Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma
- PMID: 27216216
- PMCID: PMC4937359
- DOI: 10.1182/blood-2016-03-705210
Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma
Abstract
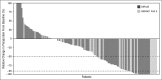
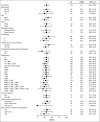
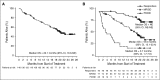
The efficacy and favorable safety profile of daratumumab monotherapy in multiple myeloma (MM) was previously reported. Here, we present an updated pooled analysis of 148 patients treated with daratumumab 16 mg/kg. Data were combined from part 2 of a first-in-human phase 1/2 study of patients who relapsed after or were refractory to ≥2 prior therapies and a phase 2 study of patients previously treated with ≥3 prior lines of therapy (including a proteasome inhibitor [PI] and an immunomodulatory drug [IMiD]) or were double refractory. Among the pooled population, patients received a median of 5 prior lines of therapy (range, 2 to 14 prior lines of therapy), and 86.5% were double refractory to a PI and an IMiD. Overall response rate was 31.1%, including 13 very good partial responses, 4 complete responses, and 3 stringent complete responses. The median duration of response was 7.6 months (95% confidence interval [CI], 5.6 to not evaluable [NE]). The median progression-free survival (PFS) and overall survival (OS) were 4.0 months (95% CI, 2.8-5.6 months) and 20.1 months (95% CI, 16.6 months to NE), respectively. When stratified by responders vs stable disease/minimal response vs progressive disease/NE, median PFS was 15.0 months (95% CI, 7.4 months to NE) vs 3.0 months (95% CI, 2.8-3.7 months) vs 0.9 months (95% CI, 0.9-1.0 months), respectively, and median OS was NE (95% CI, NE to NE) vs 18.5 months (95% CI, 15.1-22.4 months) vs 3.7 months (95% CI, 1.7-7.6 months), respectively. No new safety signals were identified. In this pooled data set, daratumumab 16 mg/kg monotherapy demonstrated rapid, deep, and durable responses, with a clinical benefit that extended to patients with stable disease or better.
© 2016 by The American Society of Hematology.
Figures

References
-
- Kastritis E, Zervas K, Symeonidis A, et al. Improved survival of patients with multiple myeloma after the introduction of novel agents and the applicability of the International Staging System (ISS): an analysis of the Greek Myeloma Study Group (GMSG). Leukemia. 2009;23(6):1152–1157. - PubMed
-
- Usmani S, Ahmadi T, Ng Y, Lam A, Potluri R, Mehra M. Analyses of real world data on overall survival in multiple myeloma patients with at least 3 prior lines of therapy including a PI and an IMiD, or double refractory to a PI and an IMiD [abstract]. Blood. 2015;126(23). Abstract 4498. - PMC - PubMed
-
- de Weers M, Tai YT, van der Veer MS, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186(3):1840–1848. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

