Oral artemisinin monotherapy removal from the private sector in Eastern Myanmar between 2012 and 2014
- PMID: 27216408
- PMCID: PMC4877749
- DOI: 10.1186/s12936-016-1292-8
Oral artemisinin monotherapy removal from the private sector in Eastern Myanmar between 2012 and 2014
Abstract
Background: In 2012 the Artemisinin Monotherapy Therapy Replacement (AMTR) project was implemented in Eastern Myanmar to increase access to subsidized, quality-assured artemisinin combination therapy (ACT) and to remove oral artemisinin monotherapy (AMT) from the private sector. The aim of this paper is to examine changes over time in the private sector anti-malarial landscape and to illustrate the value of complementary interventions in the context of a national ACT subsidy.
Methods: Three rounds of cross-sectional malaria medicine outlet surveys were conducted, in 2012, 2013 and 2014. Project intervention areas were selected from the Myanmar Artemisinin Resistance Containment (MARC) area. Provider detailing was implemented in these selected areas. Comparison areas were selected outside of this catchment area, from townships in close proximity to the MARC framework. Within each domain, multi-staged sampling was used to select areas for the survey. Outlets with the potential to sell or distribute anti-malarials in the private sector were screened for eligibility.
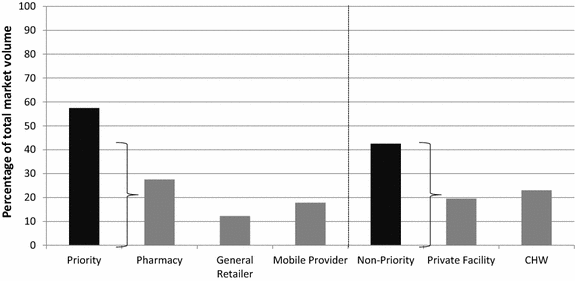
Results: The total number of outlets approached for an interview was as follows in the intervention and comparison areas, respectively: 2012, N = 2046 and 1612; 2013, N = 1636 and 1884; 2014, N = 2939 and 2941. The percentage of pharmacies, general retailers and mobile providers (classed as 'priority outlets') with oral AMT in stock on the day of the survey decreased over time in the intervention areas (2012 = 68 %; 2013 = 48 %; 2014 = 10 %). Conversely, quality-assured ACT availability increased among these outlets (2012 = 4 %; 2013 = 62 %; 2014 = 79 %). Relative oral AMT market share among priority outlets also decreased over time (2012 = 44 %; 2013 = 18 %; 2014 = 14 %), while market share of quality-assured ACT increased (2012 = 3 %; 2013 = 59 %; 2014 = 51 %). Among priority outlets in the comparison area, similar trends were observed, though changes over time were less substantial compared to the intervention area. Other outlet types (community health workers and health facilities) performed relatively well over time though modest improvements were also observed.
Conclusion: The findings point to the successful design and implementation of a strategy to rapidly remove oral AMT from pharmacies, general retailers and mobile providers and to replace its use with quality-assured ACT. The evidence also highlights the importance of supporting interventions in the context of a high-level subsidy.
Keywords: Antimalarial drug resistance; Artemisinin combination therapy; Malaria elimination; Oral artemisinin monotherapy; Outlet survey; Subsidy.
Figures







References
-
- WHO . World Malaria Report. Geneva: World Health Organization; 2015.
-
- WHO. Global Malaria Programme . Stop the marketing of oral artemisinin-based monotherapies: The World Health Organization (WHO) urges regulatory measures to stop marketing of oral artemisinin-based monotherapies and to promote access to artemisinin-based combination therapies (ACTs) Geneva: World Health Organization; 2010.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

