Whole exome sequencing links dental tumor to an autosomal-dominant mutation in ANO5 gene associated with gnathodiaphyseal dysplasia and muscle dystrophies
- PMID: 27216912
- PMCID: PMC4877638
- DOI: 10.1038/srep26440
Whole exome sequencing links dental tumor to an autosomal-dominant mutation in ANO5 gene associated with gnathodiaphyseal dysplasia and muscle dystrophies
Abstract
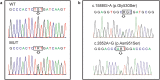
Tumors of the jaws may represent different human disorders and frequently associate with pathologic bone fractures. In this report, we analyzed two affected siblings from a family of Russian origin, with a history of dental tumors of the jaws, in correspondence to original clinical diagnosis of cementoma consistent with gigantiform cementoma (GC, OMIM: 137575). Whole exome sequencing revealed the heterozygous missense mutation c.1067G > A (p.Cys356Tyr) in ANO5 gene in these patients. To date, autosomal-dominant mutations have been described in the ANO5 gene for gnathodiaphyseal dysplasia (GDD, OMIM: 166260), and multiple recessive mutations have been described in the gene for muscle dystrophies (OMIM: 613319, 611307); the same amino acid (Cys) at the position 356 is mutated in GDD. These genetic data and similar clinical phenotypes demonstrate that the GC and GDD likely represent the same type of bone pathology. Our data illustrate the significance of mutations in single amino-acid position for particular bone tissue pathology. Modifying role of genetic variations in another gene on the severity of the monogenic trait pathology is also suggested. Finally, we propose the model explaining the tissue-specific manifestation of clinically distant bone and muscle diseases linked to mutations in one gene.
Figures


References
-
- Akasaka Y., Nakajima T., KoyamaK, Furuya K. & Mitsuka Y. [Familial cases of a new systemic bone disease, hereditary gnatho-diaphyseal sclerosis]. Nihon Seikeigeka Gakkai zasshi 43, 381–394 (1969). - PubMed
-
- Riminucci M. et al. Gnathodiaphyseal Dysplasia: A Syndrome of Fibro-Osseous Lesions of Jawbones, Bone Fragility, and Long Bone Bowing. J. Bone Miner. Res. 16, 1710–1718 (2001). - PubMed
-
- Young S. K., Markowitz N. R., Sullivan S., Seale T. W. & Hirschi R. Familial gigantiform cementoma: Classification and presentation of a large pedigree. Oral Surgery, Oral Med. Oral Pathol. 68, 740–747 (1989). - PubMed
-
- Rossbach H.-C., Letson D., Lacson A., Ruas E. & Salazar P. Familial gigantiform cementoma with brittle bone disease, pathologic fractures, and osteosarcoma: A possible explanation of an ancient mystery. Pediatr. Blood Cancer 44, 390–396 (2005). - PubMed
-
- Moshref M. et al. Autosomal dominant gigantiform cementoma associated with bone fractures. Am. J. Med. Genet. Part A 146A, 644–648 (2008). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

