Albumin nanostructures as advanced drug delivery systems
- PMID: 27216915
- PMCID: PMC5063715
- DOI: 10.1080/17425247.2016.1193149
Albumin nanostructures as advanced drug delivery systems
Abstract
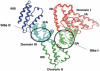
One of the biggest impacts that the nanotechnology has made on medicine and biology, has been in the area of drug delivery systems (DDSs). Many drugs suffer from serious problems concerning insolubility, instability in biological environments, poor uptake into cells and tissues, sub-optimal selectivity for targets and unwanted side effects. Nanocarriers can be designed as DDSs to overcome many of these drawbacks. One of the most versatile building blocks to prepare these nanocarriers is the ubiquitous, readily available and inexpensive protein, serum albumin. Areas covered: This review covers the use of different types of albumin (human, bovine, rat, and chicken egg) to prepare nanoparticle and microparticle-based structures to bind drugs. Various methods have been used to modify the albumin structure. A range of targeting ligands can be attached to the albumin that can be recognized by specific cell receptors that are expressed on target cells or tissues. Expert opinion: The particular advantages of albumin used in DDSs include ready availability, ease of chemical modification, good biocompatibility, and low immunogenicity. The regulatory approvals that have been received for several albumin-based therapeutic agents suggest that this approach will continue to be successfully explored.
Keywords: Human serum albumin; bovine serum albumin; drug delivery systems; nanoparticles; ovalbumin.
Figures





References
-
- Nicolas J, Mura S, Brambilla D, et al. Design, functionalization strategies and biomedical applications of targeted biodegradable/ biocompatible polymer-based nanocarriers for drug delivery. Chem Soc Rev. 2013;42(3):1147–1235. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
