Identification and functional analysis of two novel connexin 50 mutations associated with autosome dominant congenital cataracts
- PMID: 27216975
- PMCID: PMC4877569
- DOI: 10.1038/srep26551
Identification and functional analysis of two novel connexin 50 mutations associated with autosome dominant congenital cataracts
Abstract
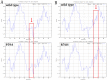
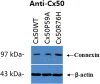
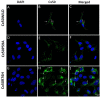
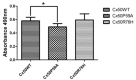
Autosomal dominant congenital cataracts (ADCC) are clinically and genetically heterogeneous diseases. The present study recruited two Chinese families with bilateral nuclear cataract or zonular pulverulent phenotype. Direct sequencing of candidate genes identified two novel missense mutations of Cx50, Cx50P59A (c.175C > G) and Cx50R76H (c.227G > A), both co-segregated well with all affected individuals. Bioinformatics analysis predicted deleterious for both mutations. Functional and cellular behaviors of wild type and mutant Cx50 examined by stably transfecting recombinant systems revealed similar protein expression levels. Protein distribution pattern by fluorescence microscopy showed that Cx50R76H localized at appositional membranes forming gap junctions with enormous cytoplasmic protein accumulation, whereas the Cx50P59A mutation was found inefficient at forming detectable plaques. Cell growth test by MTT assay showed that induction of Cx50P59A decreased cell viability. Our study constitutes the first report that the Cx50P59A and Cx50R76H mutations are associated with ADCC and expands the mutation spectrum of Cx50 in association with congenital cataracts. The genetic, cellular, and functional data suggest that the altered intercellular communication governed by mutated Cx50 proteins may act as the molecular mechanism underlying ADCC, which further confirms the role of Cx50 in the maintenance of human lens transparency.
Figures


Similar articles
-
Identification and preliminary functional analysis of two novel congenital cataract associated mutations of Cx46 and Cx50.Ophthalmic Genet. 2019 Oct;40(5):428-435. doi: 10.1080/13816810.2019.1675179. Epub 2019 Oct 16. Ophthalmic Genet. 2019. PMID: 31618082
-
A novel connexin50 mutation associated with congenital nuclear pulverulent cataracts.J Med Genet. 2008 Mar;45(3):155-60. doi: 10.1136/jmg.2007.051029. Epub 2007 Nov 15. J Med Genet. 2008. PMID: 18006672 Free PMC article.
-
A Missense Mutation in GJA8 Encoding Connexin 50 in a Chinese Pedigree with Autosomal Dominant Congenital Cataract.Tohoku J Exp Med. 2018 Feb;244(2):105-111. doi: 10.1620/tjem.244.105. Tohoku J Exp Med. 2018. PMID: 29434075
-
Gap junctions or hemichannel-dependent and independent roles of connexins in cataractogenesis and lens development.Curr Mol Med. 2010 Dec;10(9):851-63. doi: 10.2174/156652410793937750. Curr Mol Med. 2010. PMID: 21091421 Free PMC article. Review.
-
Focus on lens connexins.BMC Cell Biol. 2017 Jan 17;18(Suppl 1):6. doi: 10.1186/s12860-016-0116-6. BMC Cell Biol. 2017. PMID: 28124626 Free PMC article. Review.
Cited by
-
GJA8-associated developmental eye disorders: a new multicentre study highlights mutational hotspots and genotype-phenotype correlations.Eur J Hum Genet. 2025 Jul;33(7):860-869. doi: 10.1038/s41431-025-01843-8. Epub 2025 Apr 30. Eur J Hum Genet. 2025. PMID: 40301690 Free PMC article.
-
New GJA8 variants and phenotypes highlight its critical role in a broad spectrum of eye anomalies.Hum Genet. 2019 Sep;138(8-9):1027-1042. doi: 10.1007/s00439-018-1875-2. Epub 2018 Feb 20. Hum Genet. 2019. PMID: 29464339
-
Inherited Congenital Cataract: A Guide to Suspect the Genetic Etiology in the Cataract Genesis.Mol Syndromol. 2017 Mar;8(2):58-78. doi: 10.1159/000455752. Epub 2017 Feb 7. Mol Syndromol. 2017. PMID: 28611546 Free PMC article. Review.
-
Global Prevalence of Severe Neonatal Jaundice among Hospital Admissions: A Systematic Review and Meta-Analysis.J Clin Med. 2023 May 29;12(11):3738. doi: 10.3390/jcm12113738. J Clin Med. 2023. PMID: 37297932 Free PMC article. Review.
-
Longitudinal study of microphthalmia in connexin 50 knockout mice using spectral-domain optical coherence tomography.Front Ophthalmol (Lausanne). 2024 May 3;4:1387961. doi: 10.3389/fopht.2024.1387961. eCollection 2024. Front Ophthalmol (Lausanne). 2024. PMID: 38984115 Free PMC article.
References
-
- Santana A. & Waiswo M. The genetic and molecular basis of congenital cataract. Arquivos Brasileiros De Oftalmologia 74, 136–142 (2011). - PubMed
-
- Goodenough D. A. The crystalline lens. A system networked by gap junctional intercellular communication. Seminars in Cell Biology 3, 49–58 (1992). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

