Next-generation proteasome inhibitor MLN9708 sensitizes breast cancer cells to doxorubicin-induced apoptosis
- PMID: 27217076
- PMCID: PMC4877646
- DOI: 10.1038/srep26456
Next-generation proteasome inhibitor MLN9708 sensitizes breast cancer cells to doxorubicin-induced apoptosis
Abstract
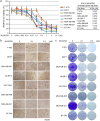
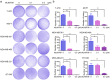
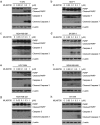
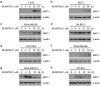
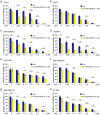
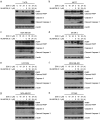
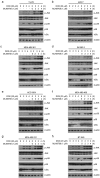
Doxorubicin (Dox), one of the most effective chemotherapy drug for cancer treatment, is limited by its severe side effects and chemoresistance. Dox induces DNA damage and leads to significant proteomic changes in the cancer cells, which makes the ubiquitin-proteasome system a potential target to enhance the efficacy of Dox therapy. The unsuccessful clinical trials of proteasome inhibitor PS-341 (bortezomib) in solid tumors led to the invention of MLN9708 (ixazomib), an orally bioavailable next-generation proteasome inhibitor with improved pharmacokinetic and pharmacodynamic features. In this preclinical study, we used eight human breast cancer cell lines, which represent the major molecular subtypes of breast cancer, to validate the cytotoxic effects of MLN9708, alone and in combination with Dox. We found that MLN9708 had cytotoxic effects, induced autophagy and MKP-1 expression, and enhanced Dox-induced apoptosis in these cell lines. MLN9708 also enhanced Dox-induced JNK and p38 phosphorylation and inhibited Dox-induced IκBα degradation. Our in vitro results suggest that MLN9708 has antitumor effects in breast cancer and can sensitize breast cancer cells to Dox treatment. This promising combination may be an effective and feasible therapeutic option for treating breast cancer and warrants clinical validation.
Figures

Similar articles
-
Second-generation proteasome inhibitor carfilzomib enhances doxorubicin-induced cytotoxicity and apoptosis in breast cancer cells.Oncotarget. 2016 Nov 8;7(45):73697-73710. doi: 10.18632/oncotarget.12048. Oncotarget. 2016. PMID: 27655642 Free PMC article.
-
Delanzomib, a novel proteasome inhibitor, sensitizes breast cancer cells to doxorubicin-induced apoptosis.Thorac Cancer. 2019 Apr;10(4):918-929. doi: 10.1111/1759-7714.13030. Epub 2019 Mar 18. Thorac Cancer. 2019. PMID: 30883017 Free PMC article.
-
TAK1 inhibitor NG25 enhances doxorubicin-mediated apoptosis in breast cancer cells.Sci Rep. 2016 Sep 7;6:32737. doi: 10.1038/srep32737. Sci Rep. 2016. PMID: 27599572 Free PMC article.
-
Ixazomib for the treatment of multiple myeloma.Expert Opin Investig Drugs. 2015;24(9):1287-98. doi: 10.1517/13543784.2015.1065250. Epub 2015 Jul 3. Expert Opin Investig Drugs. 2015. PMID: 26138345 Review.
-
[Proteasome inhibitor].Nihon Rinsho. 2014 Jun;72(6):1125-9. Nihon Rinsho. 2014. PMID: 25016815 Review. Japanese.
Cited by
-
Second-generation proteasome inhibitor carfilzomib enhances doxorubicin-induced cytotoxicity and apoptosis in breast cancer cells.Oncotarget. 2016 Nov 8;7(45):73697-73710. doi: 10.18632/oncotarget.12048. Oncotarget. 2016. PMID: 27655642 Free PMC article.
-
p62 Promotes the Mitochondrial Localization of p53 through Its UBA Domain and Participates in Regulating the Sensitivity of Ovarian Cancer Cells to Cisplatin.Int J Mol Sci. 2022 Mar 18;23(6):3290. doi: 10.3390/ijms23063290. Int J Mol Sci. 2022. PMID: 35328718 Free PMC article.
-
Rational drug combination design in patient-derived avatars reveals effective inhibition of hepatocellular carcinoma with proteasome and CDK inhibitors.J Exp Clin Cancer Res. 2022 Aug 15;41(1):249. doi: 10.1186/s13046-022-02436-9. J Exp Clin Cancer Res. 2022. PMID: 35971164 Free PMC article.
-
Screening and identification of HTNVpv entry inhibitors with high-throughput pseudovirus-based chemiluminescence.Virol Sin. 2022 Aug;37(4):531-537. doi: 10.1016/j.virs.2022.04.015. Epub 2022 May 2. Virol Sin. 2022. PMID: 35513270 Free PMC article.
-
MiR-770 suppresses the chemo-resistance and metastasis of triple negative breast cancer via direct targeting of STMN1.Cell Death Dis. 2018 Jan 11;9(1):14. doi: 10.1038/s41419-017-0030-7. Cell Death Dis. 2018. PMID: 29323124 Free PMC article.
References
-
- Hortobagyi G. N. Developments in chemotherapy of breast cancer. Cancer 88, 3073–3079 (2000). - PubMed
-
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast cancer (Version 3.2015) (2015).
-
- Tacar O., Sriamornsak P. & Dass C. R. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 65, 157–170 (2013). - PubMed
-
- Swift L. P., Rephaeli A., Nudelman A., Phillips D. R. & Cutts S. M. Doxorubicin-DNA adducts induce a non-topoisomerase II-mediated form of cell death. Cancer Res. 66, 4863–4871 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

