Conditional ablation of neuroligin-1 in CA1 pyramidal neurons blocks LTP by a cell-autonomous NMDA receptor-independent mechanism
- PMID: 27217145
- PMCID: PMC5122464
- DOI: 10.1038/mp.2016.80
Conditional ablation of neuroligin-1 in CA1 pyramidal neurons blocks LTP by a cell-autonomous NMDA receptor-independent mechanism
Abstract
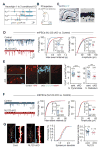
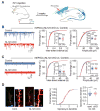
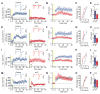
Neuroligins are postsynaptic cell-adhesion molecules implicated in autism and other neuropsychiatric disorders. Despite extensive work, the role of neuroligins in synapse function and plasticity, especially N-methyl-d-aspartate (NMDA) receptor (NMDAR)-dependent long-term potentiation (LTP), remains unclear. To establish which synaptic functions unequivocally require neuroligins, we analyzed single and triple conditional knockout (cKO) mice for all three major neuroligin isoforms (NL1-NL3). We inactivated neuroligins by stereotactic viral expression of Cre-recombinase in hippocampal CA1 region pyramidal neurons at postnatal day 0 (P0) or day 21 (P21) and measured synaptic function, synaptic plasticity and spine numbers in acute hippocampal slices 2-3 weeks later. Surprisingly, we find that ablation of neuroligins in newborn or juvenile mice only modestly impaired basal synaptic function in hippocampus and caused no alteration in postsynaptic spine numbers. However, triple cKO of NL1-NL3 or single cKO of NL1 impaired NMDAR-mediated excitatory postsynaptic currents and abolished NMDAR-dependent LTP. Strikingly, the NL1 cKO also abolished LTP elicited by activation of L-type Ca2+-channels during blockade of NMDARs. These findings demonstrate that neuroligins are generally not essential for synapse formation in CA1 pyramidal neurons but shape synaptic properties and that NL1 specifically is required for LTP induced by postsynaptic Ca2+-elevations, a function which may contribute to the pathophysiological role of neuroligins in brain disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Neuroligins Are Selectively Essential for NMDAR Signaling in Cerebellar Stellate Interneurons.J Neurosci. 2016 Aug 31;36(35):9070-83. doi: 10.1523/JNEUROSCI.1356-16.2016. J Neurosci. 2016. PMID: 27581450 Free PMC article.
-
Neuroligin-1 Signaling Controls LTP and NMDA Receptors by Distinct Molecular Pathways.Neuron. 2019 May 8;102(3):621-635.e3. doi: 10.1016/j.neuron.2019.02.013. Epub 2019 Mar 11. Neuron. 2019. PMID: 30871858 Free PMC article.
-
Unique versus Redundant Functions of Neuroligin Genes in Shaping Excitatory and Inhibitory Synapse Properties.J Neurosci. 2017 Jul 19;37(29):6816-6836. doi: 10.1523/JNEUROSCI.0125-17.2017. Epub 2017 Jun 12. J Neurosci. 2017. PMID: 28607166 Free PMC article.
-
Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors.Crit Rev Neurobiol. 2006;18(1-2):71-84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. Crit Rev Neurobiol. 2006. PMID: 17725510 Review.
-
Muscarinic Receptors, from Synaptic Plasticity to its Role in Network Activity.Neuroscience. 2021 Feb 21;456:60-70. doi: 10.1016/j.neuroscience.2020.04.005. Epub 2020 Apr 8. Neuroscience. 2021. PMID: 32278062 Review.
Cited by
-
Towards an Understanding of Synapse Formation.Neuron. 2018 Oct 24;100(2):276-293. doi: 10.1016/j.neuron.2018.09.040. Neuron. 2018. PMID: 30359597 Free PMC article. Review.
-
SALM1 controls synapse development by promoting F-actin/PIP2-dependent Neurexin clustering.EMBO J. 2019 Sep 2;38(17):e101289. doi: 10.15252/embj.2018101289. Epub 2019 Aug 1. EMBO J. 2019. PMID: 31368584 Free PMC article.
-
Neuroligin-1 mediates presynaptic maturation through brain-derived neurotrophic factor signaling.BMC Biol. 2021 Sep 27;19(1):215. doi: 10.1186/s12915-021-01145-7. BMC Biol. 2021. PMID: 34579720 Free PMC article.
-
Structure, function, and pathology of Neurexin-3.Genes Dis. 2022 Apr 30;10(5):1908-1919. doi: 10.1016/j.gendis.2022.04.008. eCollection 2023 Sep. Genes Dis. 2022. PMID: 37492720 Free PMC article. Review.
-
Differential Properties of the Synaptogenic Activities of the Neurexin Ligands Neuroligin1 and LRRTM2.Front Mol Neurosci. 2019 Nov 8;12:269. doi: 10.3389/fnmol.2019.00269. eCollection 2019. Front Mol Neurosci. 2019. PMID: 31780894 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

