Cold-inducible RNA-binding protein causes endothelial dysfunction via activation of Nlrp3 inflammasome
- PMID: 27217302
- PMCID: PMC4877585
- DOI: 10.1038/srep26571
Cold-inducible RNA-binding protein causes endothelial dysfunction via activation of Nlrp3 inflammasome
Abstract
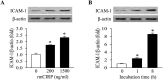
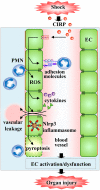
Cold-inducible RNA-binding protein (CIRP) is a damage-associated molecular pattern (DAMP) molecule which stimulates proinflammatory cytokine release in hemorrhage and sepsis. Under these medical conditions, disruption of endothelial homeostasis and barrier integrity, typically induced by proinflammatory cytokines, is an important factor contributing to morbidity and mortality. However, the role of CIRP in causing endothelial dysfunction has not been investigated. In this study, we show that intravenous injection of recombinant murine CIRP (rmCIRP) in C57BL/6 mice causes lung injury, evidenced by vascular leakage, edema, increased leukocyte infiltration and cytokine production in the lung tissue. The CIRP-induced lung damage is accompanied with endothelial cell (EC) activation marked by upregulation of cell-surface adhesion molecules E-selectin and ICAM-1. Using in vitro primary mouse lung vascular ECs (MLVECs), we demonstrate that rmCIRP treatment directly increases the ICAM-1 protein expression and activates NAD(P)H oxidase in MLVECs. Importantly, CIRP stimulates the assembly and activation of Nlrp3 inflammasome in MLVECs accompanied with caspase-1 activation, IL-1β release and induction of proinflammatory cell death pyroptosis. Finally, our study demonstrates CIRP-induced EC pyroptosis in the lungs of C57BL/6 mice for the first time. Taken together, the released CIRP in shock can directly activate ECs and induce EC pyroptosis to cause lung injury.
Figures

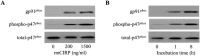


References
-
- Schouten M., Wiersinga W. J., Levi M. & van der Poll T. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol. 83, 536–545 (2008). - PubMed
-
- Diebel L. N., Liberati D. M., Ledgerwood A. M. & Lucas C. E. Changes in lymph proteome induced by hemorrhagic shock: the appearance of damage-associated molecular patterns. J Trauma Acute Care Surg. 73, 41–51 (2012). - PubMed
-
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 454, 428–435 (2008). - PubMed
-
- Kaczorowski D. J., Mollen K. P., Edmonds R. & Billiar T. R. Early events in the recognition of danger signals after tissue injury. J Leukoc Biol. 83, 546–552 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

