Genetic and phenotypic characterization of complex hereditary spastic paraplegia
- PMID: 27217339
- PMCID: PMC4939695
- DOI: 10.1093/brain/aww111
Genetic and phenotypic characterization of complex hereditary spastic paraplegia
Abstract
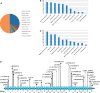
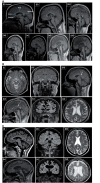
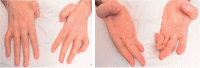
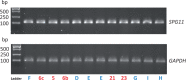
The hereditary spastic paraplegias are a heterogeneous group of degenerative disorders that are clinically classified as either pure with predominant lower limb spasticity, or complex where spastic paraplegia is complicated with additional neurological features, and are inherited in autosomal dominant, autosomal recessive or X-linked patterns. Genetic defects have been identified in over 40 different genes, with more than 70 loci in total. Complex recessive spastic paraplegias have in the past been frequently associated with mutations in SPG11 (spatacsin), ZFYVE26/SPG15, SPG7 (paraplegin) and a handful of other rare genes, but many cases remain genetically undefined. The overlap with other neurodegenerative disorders has been implied in a small number of reports, but not in larger disease series. This deficiency has been largely due to the lack of suitable high throughput techniques to investigate the genetic basis of disease, but the recent availability of next generation sequencing can facilitate the identification of disease-causing mutations even in extremely heterogeneous disorders. We investigated a series of 97 index cases with complex spastic paraplegia referred to a tertiary referral neurology centre in London for diagnosis or management. The mean age of onset was 16 years (range 3 to 39). The SPG11 gene was first analysed, revealing homozygous or compound heterozygous mutations in 30/97 (30.9%) of probands, the largest SPG11 series reported to date, and by far the most common cause of complex spastic paraplegia in the UK, with severe and progressive clinical features and other neurological manifestations, linked with magnetic resonance imaging defects. Given the high frequency of SPG11 mutations, we studied the autophagic response to starvation in eight affected SPG11 cases and control fibroblast cell lines, but in our restricted study we did not observe correlations between disease status and autophagic or lysosomal markers. In the remaining cases, next generation sequencing was carried out revealing variants in a number of other known complex spastic paraplegia genes, including five in SPG7 (5/97), four in FA2H (also known as SPG35) (4/97) and two in ZFYVE26/SPG15 Variants were identified in genes usually associated with pure spastic paraplegia and also in the Parkinson's disease-associated gene ATP13A2, neuronal ceroid lipofuscinosis gene TPP1 and the hereditary motor and sensory neuropathy DNMT1 gene, highlighting the genetic heterogeneity of spastic paraplegia. No plausible genetic cause was identified in 51% of probands, likely indicating the existence of as yet unidentified genes.
Keywords: Parkinson’s disease; SPG11; gene; hereditary spastic paraplegia; mutation.
© The Author (2016). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures


References
-
- Anheim M, Lagier-Tourenne C, Stevanin G, Fleury M, Durr A, Namer IJ, et al. . SPG11 spastic paraplegia. A new cause of juvenile parkinsonism . J Neurol 2009a. ; 256 : 104 – 8 . - PubMed
-
- Anheim M, Lagier-Tourenne C, Stevanin G, Fleury M, Durr A, Namer IJ, et al. . SPG11 spastic paraplegia. A new cause of juvenile parkinsonism . J Neurol 2009b. ; 256 : 104 – 8 . - PubMed
-
- Bauer P, Winner B, Schule R, Bauer C, Hafele V, Hehr U, et al. . Identification of a heterozygous genomic deletion in the spatacsin gene in SPG11 patients using high-resolution comparative genomic hybridization . Neurogenetics 2009. ; 10 : 43 – 8 . - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

