Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial
- PMID: 27217345
- PMCID: PMC4922848
- DOI: 10.5966/sctm.2015-0245
Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial
Abstract
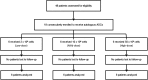
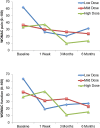
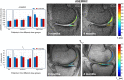
: Osteoarthritis (OA) is the most widespread musculoskeletal disorder in adults. It leads to cartilage damage associated with subchondral bone changes and synovial inflammation, causing pain and disability. The present study aimed at evaluating the safety of a dose-escalation protocol of intra-articular injected adipose-derived stromal cells (ASCs) in patients with knee OA, as well as clinical efficacy as secondary endpoint. A bicentric, uncontrolled, open phase I clinical trial was conducted in France and Germany with regulatory agency approval for ASC expansion procedure in both countries. From April 2012 to December 2013, 18 consecutive patients with symptomatic and severe knee OA were treated with a single intra-articular injection of autologous ASCs. The study design consisted of three consecutive cohorts (six patients each) with dose escalation: low dose (2 × 10(6) cells), medium dose (10 × 10(6)), and high dose (50 × 10(6)). The primary outcome parameter was safety evaluated by recording adverse events throughout the trial, and secondary parameters were pain and function subscales of the Western Ontario and McMaster Universities Arthritis Index. After 6 months of follow-up, the procedure was found to be safe, and no serious adverse events were reported. Four patients experienced transient knee joint pain and swelling after local injection. Interestingly, patients treated with low-dose ASCs experienced significant improvements in pain levels and function compared with baseline. Our data suggest that the intra-articular injection of ASCs is a safe therapeutic alternative to treat severe knee OA patients. A placebo-controlled double-blind phase IIb study is being initiated to assess clinical and structural efficacy.
Significance: Although this phase I study included a limited number of patients without a placebo arm, it showed that local injection of autologous adipose-derived stem cells was safe and well tolerated in patients with knee osteoarthritis. This study also provides encouraging preliminary evidence of efficacy. Larger and controlled long-term studies are now mandatory to confirm whether this new strategy of cell therapy can improve pain and induce structural benefit in osteoarthritis.
Trial registration: ClinicalTrials.gov NCT01585857.
Keywords: Adipose mesenchymal stromal cells; Intra-articular injection; Osteoarthritis; Phase I clinical trial; Regenerative medicine; Therapeutic potential.
©AlphaMed Press.
Figures

References
-
- Jorgensen C, Djouad F, Bouffi C, et al. Multipotent mesenchymal stromal cells in articular diseases. Best Pract Res Clin Rheumatol. 2008;22:269–284. - PubMed
-
- ter Huurne M, Schelbergen R, Blattes R, et al. Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum. 2012;64:3604–3613. - PubMed
-
- Bura A, Planat-Benard V, Bourin P, et al. Phase I trial: The use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy. 2014;16:245–257. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

