Concise Review: Pancreatic Cancer and Bone Marrow-Derived Stem Cells
- PMID: 27217346
- PMCID: PMC4922853
- DOI: 10.5966/sctm.2015-0291
Concise Review: Pancreatic Cancer and Bone Marrow-Derived Stem Cells
Abstract
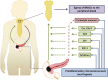
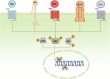
Pancreatic adenocarcinoma remains one of the most challenging diseases of modern gastroenterology, and, even though considerable effort has been put into understanding its pathogenesis, the exact molecular mechanisms underlying the development and/or systemic progression of this malignancy still remain unclear. Recently, much attention has been paid to the potential role of bone marrow-derived stem cells (BMSCs) in this malignancy. Hence, herein, we comprehensively review the most recent discoveries and current achievements and concepts in this field. Specifically, we discuss the significance of identifying pancreatic cancer stem cells and novel therapeutic approaches involving molecular interference of their metabolism. We also describe advances in the current understanding of the biochemical and molecular mechanisms responsible for BMSC mobilization during pancreatic cancer development and systemic spread. Finally, we summarize experimental, translational, and/or clinical evidence regarding the contribution of bone marrow-derived mesenchymal stem cells, endothelial progenitor cells, hematopoietic stem/progenitor cells, and pancreatic stellate cells in pancreatic cancer development/progression. We also present their potential therapeutic value for the treatment of this deadly malignancy in humans.
Significance: Different bone marrow-derived stem cell populations contribute to the development and/or progression of pancreatic cancer, and they might also be a promising "weapon" that can be used for anticancer treatments in humans. Even though the exact role of these stem cells in pancreatic cancer development and/or progression in humans still remains unclear, this concept continues to drive a completely novel scientific avenue in pancreatic cancer research and gives rise to innovative ideas regarding novel therapeutic modalities that can be safely offered to patients.
Keywords: Bone marrow; Cancer; Pancreas; Stem cells.
©AlphaMed Press.
Figures
References
-
- Maisonneuve P, Lowenfels AB. Risk factors for pancreatic cancer: A summary review of meta-analytical studies. Int J Epidemiol. 2015;44:186–198. - PubMed
-
- Real FX, Cibrián-Uhalte E, Martinelli P. Pancreatic cancer development and progression: Remodeling the model. Gastroenterology. 2008;135:724–728. - PubMed
-
- Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. - PubMed
-
- Abel EV, Simeone DM. Biology and clinical applications of pancreatic cancer stem cells. Gastroenterology. 2013;144:1241–1248. - PubMed
-
- Zhan HX, Xu JW, Wu D, et al. Pancreatic cancer stem cells: New insight into a stubborn disease. Cancer Lett. 2015;357:429–437. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

