Developmental cell death programs license cytotoxic cells to eliminate histocompatible partners
- PMID: 27217570
- PMCID: PMC4988592
- DOI: 10.1073/pnas.1606276113
Developmental cell death programs license cytotoxic cells to eliminate histocompatible partners
Abstract
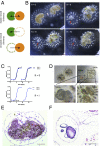
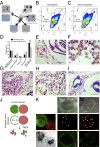
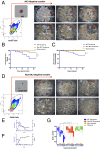
In a primitive chordate model of natural chimerism, one chimeric partner is often eliminated in a process of allogeneic resorption. Here, we identify the cellular framework underlying loss of tolerance to one partner within a natural Botryllus schlosseri chimera. We show that the principal cell type mediating chimeric partner elimination is a cytotoxic morula cell (MC). Proinflammatory, developmental cell death programs render MCs cytotoxic and, in collaboration with activated phagocytes, eliminate chimeric partners during the "takeover" phase of blastogenic development. Among these genes, the proinflammatory cytokine IL-17 enhances cytotoxicity in allorecognition assays. Cellular transfer of FACS-purified MCs from allogeneic donors into recipients shows that the resorption response can be adoptively acquired. Transfer of 1 × 10(5) allogeneic MCs eliminated 33 of 78 (42%) recipient primary buds and 20 of 76 (20.5%) adult parental adult organisms (zooids) by 14 d whereas transfer of allogeneic cell populations lacking MCs had only minimal effects on recipient colonies. Furthermore, reactivity of transferred cells coincided with the onset of developmental-regulated cell death programs and disproportionately affected developing tissues within a chimera. Among chimeric partner "losers," severe developmental defects were observed in asexually propagating tissues, reflecting a pathologic switch in gene expression in developmental programs. These studies provide evidence that elimination of one partner in a chimera is an immune cell-based rejection that operates within histocompatible pairs and that maximal allogeneic responses involve the coordination of both phagocytic programs and the "arming" of cytotoxic cells.
Keywords: apoptosis; histocompatibility; inflammation; innate immunity; macrophages.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Scofield VL, Schlumpberger JM, Weissman IL. Colony specificity in the colonial tunicate Botryllus and the origins of vertebrate immunity. Am Zool. 1982;22(4):783–794.
-
- Hirose E, Saito Y, Watanabe H. A new type of the manifestation of colony specificity in the compound ascidian, Botrylloides violaceus Oka. Biol Bull. 1988;175(2):240–245.
-
- Rinkevich B, Weissman IL. Allogeneic resorption in colonial protochordates: Consequences of nonself recognition. Dev Comp Immunol. 1992;16(4):275–286. - PubMed
-
- Rinkevich B, Weissman IL. A long-term study on fused subclones in the ascidian Botryllus schlosseri: The resorption phenomenon (Protochordata: Tunicata) J Zool (Lond) 1987;213(4):717–733.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

