CSF-1R-Dependent Lethal Hepatotoxicity When Agonistic CD40 Antibody Is Given before but Not after Chemotherapy
- PMID: 27217585
- PMCID: PMC4912938
- DOI: 10.4049/jimmunol.1600146
CSF-1R-Dependent Lethal Hepatotoxicity When Agonistic CD40 Antibody Is Given before but Not after Chemotherapy
Abstract
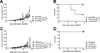
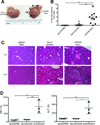
Cancer immunotherapies are increasingly effective in the clinic, especially immune checkpoint blockade delivered to patients who have T cell-infiltrated tumors. Agonistic CD40 mAb promotes stromal degradation and, in combination with chemotherapy, drives T cell infiltration and de novo responses against tumors, rendering resistant tumors susceptible to current immunotherapies. Partnering anti-CD40 with different treatments is an attractive approach for the next phase of cancer immunotherapies, with a number of clinical trials using anti-CD40 combinations ongoing, but the optimal therapeutic regimens with anti-CD40 are not well understood. Pancreatic ductal adenocarcinoma (PDA) is classically resistant to immunotherapy and lacks baseline T cell infiltration. In this study, we used a tumor cell line derived from a genetically engineered mouse model of PDA to investigate alterations in the sequence of anti-CD40 and chemotherapy as an approach to enhance pharmacological delivery of chemotherapy. Unexpectedly, despite our previous studies showing anti-CD40 treatment after chemotherapy is safe in both mice and patients with PDA, we report in this article that anti-CD40 administration <3 d in advance of chemotherapy is lethal in more than half of treated C57BL/6 mice. Anti-CD40 treatment 2 or 3 d before chemotherapy resulted in significantly increased populations of both activated myeloid cells and macrophages and lethal hepatotoxicity. Liver damage was fully abrogated when macrophage activation was blocked using anti-CSF-1R mAb. These studies highlight the dual nature of CD40 in activating both macrophages and T cell responses, and the need for preclinical investigation of optimal anti-CD40 treatment regimens for safe design of clinical trials.
Copyright © 2016 by The American Association of Immunologists, Inc.
Figures



References
-
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366:2455–2465. - PMC - PubMed
-
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. - PMC - PubMed
-
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A, investigators K- Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015;372:2521–2532. - PubMed
-
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

