Matrix metalloproteinases-2/9-sensitive peptide-conjugated polymer micelles for site-specific release of drugs and enhancing tumor accumulation: preparation and in vitro and in vivo evaluation
- PMID: 27217744
- PMCID: PMC4853011
- DOI: 10.2147/IJN.S101030
Matrix metalloproteinases-2/9-sensitive peptide-conjugated polymer micelles for site-specific release of drugs and enhancing tumor accumulation: preparation and in vitro and in vivo evaluation
Abstract
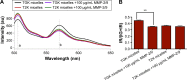
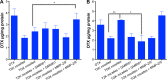
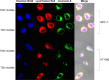
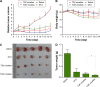
Since elevated expression of matrix metalloproteinase (MMP)-2 and MMP-9 is commonly observed in several malignant tumors, MMPs have been widely reported as key factors in the design of drug delivery systems. Several strategies have been proposed to develop MMPs-responsive nanoparticles to deliver chemotherapeutics to malignant solid tumors. A stimuli-responsive drug delivery system, which could be cleaved by MMPs, was proposed in this study. By inserting an MMP-2/9 cleavable oligopeptide GPVGLIGK-NH2 (GK8) as spacer between α-tocopherol succinate (α-TOS) and methoxy-polyethylene glycol molecular weight (MW 2000 Da) activated by N-hydroxysuccinimide (mPEG2K-NHS), mPEG2K-GK8-α-TOS (TGK) was synthesized as the primary ingredient for MMP-2/9-sensitive micelles composed of d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) and TGK (n:n =40:60, TGK micelles). mPEG2K-α-TOS (T2K) was similarly synthesized as nonsensitive control. The TGK micelles showed better stability than nonsensitive micelles composed of TPGS and T2K (n:n =40:60, T2K micelles) owing to the inserted peptide. Fluorescence resonance energy transfer results indicated that TGK micelles could be successfully cleaved by MMP-2/9. Effective drug release was demonstrated in the presence of collagenase type IV, a mixture of MMP-2 and MMP-9. Compared with nonsensitive micelles, docetaxel (DTX)-loaded TGK micelles showed a fold higher cellular uptake in HT1080 cells. While the half-maximal inhibitory concentration (IC50) of TGK and T2K micelles were similar (P>0.05) in MCF-7 cells (MMP-2/9 underexpression), the IC50 values of the aforementioned micelles were 0.064±0.006 and 0.122±0.009 μg/mL, respectively, in HT1080 cells (MMP-2/9 overexpression). The MMP-2/9-sensitive micelles also demonstrated desired tumor targeting and accumulation ability in vivo. The results of in vivo antitumor effect evaluation indicate that TGK micelles are potent against solid tumors while maintaining minimum systemic toxicity compared with T2K micelles and DTX.
Keywords: FRET; TPGS; matrix metalloproteinases; nanocarriers; stimuli responsive.
Figures






Similar articles
-
Redox-responsive polymeric micelles formed by conjugating gambogic acid with bioreducible poly(amido amine)s for the co-delivery of docetaxel and MMP-9 shRNA.Acta Biomater. 2018 Mar 1;68:137-153. doi: 10.1016/j.actbio.2017.12.028. Epub 2017 Dec 26. Acta Biomater. 2018. PMID: 29288085
-
Enhanced anticancer activity in vitro and in vivo of luteolin incorporated into long-circulating micelles based on DSPE-PEG2000 and TPGS.J Pharm Pharmacol. 2016 Oct;68(10):1290-8. doi: 10.1111/jphp.12598. Epub 2016 Jul 27. J Pharm Pharmacol. 2016. PMID: 27465923
-
Formulation of Docetaxel by folic acid-conjugated d-α-tocopheryl polyethylene glycol succinate 2000 (Vitamin E TPGS(2k)) micelles for targeted and synergistic chemotherapy.Biomaterials. 2011 Jun;32(16):4058-66. doi: 10.1016/j.biomaterials.2011.02.022. Biomaterials. 2011. PMID: 21396707
-
Current trends in the use of vitamin E-based micellar nanocarriers for anticancer drug delivery.Expert Opin Drug Deliv. 2017 Jun;14(6):715-726. doi: 10.1080/17425247.2016.1229300. Epub 2016 Sep 6. Expert Opin Drug Deliv. 2017. PMID: 27560621 Review.
-
Recent advances in TPGS-based nanoparticles of docetaxel for improved chemotherapy.Int J Pharm. 2017 Aug 30;529(1-2):506-522. doi: 10.1016/j.ijpharm.2017.07.018. Epub 2017 Jul 12. Int J Pharm. 2017. PMID: 28711640 Review.
Cited by
-
Protease-triggered bioresponsive drug delivery for the targeted theranostics of malignancy.Acta Pharm Sin B. 2021 Aug;11(8):2220-2242. doi: 10.1016/j.apsb.2021.01.017. Epub 2021 Jan 24. Acta Pharm Sin B. 2021. PMID: 34522585 Free PMC article. Review.
-
Recent Advances and Trends in Chemical CPP-Drug Conjugation Techniques.Molecules. 2021 Mar 13;26(6):1591. doi: 10.3390/molecules26061591. Molecules. 2021. PMID: 33805680 Free PMC article. Review.
-
Protective effects on myocardial infarction model: delivery of schisandrin B using matrix metalloproteinase-sensitive peptide-modified, PEGylated lipid nanoparticles.Int J Nanomedicine. 2017 Sep 26;12:7121-7130. doi: 10.2147/IJN.S141549. eCollection 2017. Int J Nanomedicine. 2017. PMID: 29026305 Free PMC article.
-
Broad-Spectrum Theranostics and Biomedical Application of Functionalized Nanomaterials.Polymers (Basel). 2022 Mar 17;14(6):1221. doi: 10.3390/polym14061221. Polymers (Basel). 2022. PMID: 35335551 Free PMC article. Review.
-
Smart Nanoplatforms Responding to the Tumor Microenvironment for Precise Drug Delivery in Cancer Therapy.Int J Nanomedicine. 2024 Jun 19;19:6253-6277. doi: 10.2147/IJN.S459710. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38911497 Free PMC article. Review.
References
-
- Liang D, Wang AT, Yang ZZ, Liu YJ, Qi XR. Enhance cancer cell recognition and overcome drug resistance using hyaluronic acid and alpha-tocopheryl succinate based multifunctional nanoparticles. Mol Pharm. 2015;12(6):2189–2202. - PubMed
-
- Li Y, Gao GH, Lee DS. Stimulus-sensitive polymeric nanoparticles and their applications as drug and gene carriers. Adv Healthc Mater. 2013;2(3):388–417. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

