Dendritic Polymers for Theranostics
- PMID: 27217829
- PMCID: PMC4876620
- DOI: 10.7150/thno.14855
Dendritic Polymers for Theranostics
Abstract
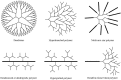
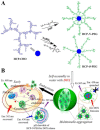
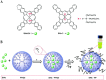
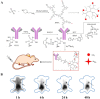
Dendritic polymers are highly branched polymers with controllable structures, which possess a large population of terminal functional groups, low solution or melt viscosity, and good solubility. Their size, degree of branching and functionality can be adjusted and controlled through the synthetic procedures. These tunable structures correspond to application-related properties, such as biodegradability, biocompatibility, stimuli-responsiveness and self-assembly ability, which are the key points for theranostic applications, including chemotherapeutic theranostics, biotherapeutic theranostics, phototherapeutic theranostics, radiotherapeutic theranostics and combined therapeutic theranostics. Up to now, significant progress has been made for the dendritic polymers in solving some of the fundamental and technical questions toward their theranostic applications. In this review, we briefly summarize how to control the structures of dendritic polymers, the theranostics-related properties derived from their structures and their theranostics-related applications.
Keywords: Dendritic polymers; theranostics.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Gao C, Yan D. Hyperbranched polymers: from synthesis to applications. Prog Polym Sci. 2004;29:183–275.
-
- Grayson SM, Frechét JM. Convergent dendrons and dendrimers: from synthesis to applications. Chem Rev. 2001;101:3819–67. - PubMed
-
- Hawker CJ, Frechét JM. Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. J Am Chem Soc. 1990;112:7638–47.
-
- Zhou Y, Huang W, Liu J, Zhu X, Yan D. Self-assembly of hyperbranched polymers and its biomedical applications. Adv Mater. 2010;22:4567–90. - PubMed
-
- Wang D, Zhao T, Zhu X, Yan D, Wang W. Bioapplications of hyperbranched polymers. Chem Soc Rev. 2015;44:4023–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

