Click Chemistry-Mediated Nanosensors for Biochemical Assays
- PMID: 27217831
- PMCID: PMC4876622
- DOI: 10.7150/thno.14856
Click Chemistry-Mediated Nanosensors for Biochemical Assays
Abstract
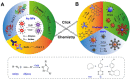
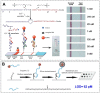
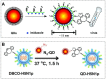
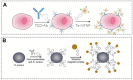
Click chemistry combined with functional nanoparticles have drawn increasing attention in biochemical assays because they are promising in developing biosensors with effective signal transformation/amplification and straightforward signal readout for clinical diagnostic assays. In this review, we focus on the latest advances of biochemical assays based on Cu (I)-catalyzed 1, 3-dipolar cycloaddition of azides and alkynes (CuAAC)-mediated nanosensors, as well as the functionalization of nanoprobes based on click chemistry. Nanoprobes including gold nanoparticles, quantum dots, magnetic nanoparticles and carbon nanomaterials are covered. We discuss the advantages of click chemistry-mediated nanosensors for biochemical assays, and give perspectives on the development of click chemistry-mediated approaches for clinical diagnosis and other biomedical applications.
Keywords: Click chemistry; bio-conjugation; nanosensor; signal amplification system..
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Sun JS, Xianyu YL, Jiang XY. Point-of-care biochemical assays using gold nanoparticle-implemented microfluidics. Chem Soc Rev. 2014;43:6239–53. - PubMed
-
- Chen WW, Li QZ, Zheng WS, Hu F, Zhang GX, Wang Z. et al. Identification of Bacteria in Water by a Fluorescent Array. Angew Chem Int Edit. 2014;53:13734–9. - PubMed
-
- Tekin HC, Gijs MAM. Ultrasensitive protein detection: a case for microfluidic magnetic bead-based assays. Lab Chip. 2013;13:4711–39. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

