The Phase Lag between Agonist-Induced Oscillatory Ca2+ and IP3 Signals Does Not Imply Causality (December 2015)
- PMID: 27218121
- PMCID: PMC4874533
The Phase Lag between Agonist-Induced Oscillatory Ca2+ and IP3 Signals Does Not Imply Causality (December 2015)
Abstract
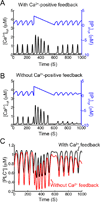
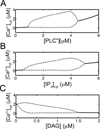
Activated phospholipase C (PLC*) generates 1,4,5-triphosphate (IP3) and diacylglycerol (DAG) from phosphatidyl inositol (PIP2). The DAG remains in the plasma membrane and co-activates conventional protein kinase C (PKC) with Ca2+. We have developed a mathematical model for the activation of the Ca2+-dependent PKC and its negative feedback on phospholipase C (PLC) and coupled it to the De Young-Keizer model for IP3 mediated Ca2+ oscillations. The model describes the cascade of reactions for the translocation of PKC to plasma membrane, and simulates activation of Ca2+ and diacylglycerol (DAG) oscillations. The model demonstrates that oscillations in Ca2+ and DAG are possible with or without a positive Ca2+ feedback on phospholipase C consistent with experiment. In many experimental studies, the timing of the peaks of the Ca2+ and IP3 oscillations have been used to suggest causality, i.e. that the IP3 oscillations cause the Ca2+ oscillations. The model is used to explore this question. To this end, the positive and negative feedback between Ca2+ and IP3 production are modulated, resulting in changes to the phase lag between the peaks in [Ca2+]cyt and [IP]cyt. The model simulates a possible experimental protocol that can be used to differentiate whether or not the positive feedback of Ca2+ on PLC is needed for the oscillations.
Keywords: calcium oscillation; computational model; diacyl glycerol (DAG); phospholipase C (PLC); protein kinase C (PKC).
Figures






References
-
- Sinnecker D, Schaefer M. Real-time analysis of phospholipase C activity during different patterns of receptor-induced Ca2+ responses in HEK293 cells. Cell calcium. 2004;35(1):29–38. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
