Multisensory integration in the developing tectum is constrained by the balance of excitation and inhibition
- PMID: 27218449
- PMCID: PMC4912350
- DOI: 10.7554/eLife.15600
Multisensory integration in the developing tectum is constrained by the balance of excitation and inhibition
Abstract
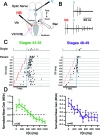
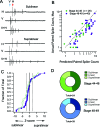
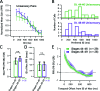
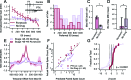
Multisensory integration (MSI) is the process that allows the brain to bind together spatiotemporally congruent inputs from different sensory modalities to produce single salient representations. While the phenomenology of MSI in vertebrate brains is well described, relatively little is known about cellular and synaptic mechanisms underlying this phenomenon. Here we use an isolated brain preparation to describe cellular mechanisms underlying development of MSI between visual and mechanosensory inputs in the optic tectum of Xenopus tadpoles. We find MSI is highly dependent on the temporal interval between crossmodal stimulus pairs. Over a key developmental period, the temporal window for MSI significantly narrows and is selectively tuned to specific interstimulus intervals. These changes in MSI correlate with developmental increases in evoked synaptic inhibition, and inhibitory blockade reverses observed developmental changes in MSI. We propose a model in which development of recurrent inhibition mediates development of temporal aspects of MSI in the tectum.
Keywords: inhibition; multisensory; neural circuit; neuroscience; tectum; visual development; visual system; xenopus.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures


Similar articles
-
A cellular mechanism for inverse effectiveness in multisensory integration.Elife. 2017 Mar 18;6:e25392. doi: 10.7554/eLife.25392. Elife. 2017. PMID: 28315524 Free PMC article.
-
Excitation and inhibition in recurrent networks mediate collision avoidance in Xenopus tadpoles.Eur J Neurosci. 2014 Sep;40(6):2948-62. doi: 10.1111/ejn.12664. Epub 2014 Jul 4. Eur J Neurosci. 2014. PMID: 24995793
-
The horizontal brain slice preparation: a novel approach for visualizing and recording from all layers of the tadpole tectum.J Neurophysiol. 2015 Jan 1;113(1):400-7. doi: 10.1152/jn.00672.2014. Epub 2014 Oct 15. J Neurophysiol. 2015. PMID: 25343786 Free PMC article.
-
Multimodality in the Collicular Pathway: Towards Compensatory Visual Processes.Cells. 2025 Apr 25;14(9):635. doi: 10.3390/cells14090635. Cells. 2025. PMID: 40358159 Free PMC article. Review.
-
The decision to move: response times, neuronal circuits and sensory memory in a simple vertebrate.Proc Biol Sci. 2019 Mar 27;286(1899):20190297. doi: 10.1098/rspb.2019.0297. Proc Biol Sci. 2019. PMID: 30900536 Free PMC article. Review.
Cited by
-
Morphology and Dendrite-Specific Synaptic Properties of Midbrain Neurons Shape Multimodal Integration.J Neurosci. 2022 Mar 30;42(13):2614-2630. doi: 10.1523/JNEUROSCI.1695-21.2022. Epub 2022 Feb 8. J Neurosci. 2022. PMID: 35135851 Free PMC article.
-
Multisensory integration enhances audiovisual responses in the Mauthner cell.Elife. 2024 Dec 5;13:RP99424. doi: 10.7554/eLife.99424. Elife. 2024. PMID: 39636208 Free PMC article.
-
Emergence of Selectivity to Looming Stimuli in a Spiking Network Model of the Optic Tectum.Front Neural Circuits. 2016 Nov 24;10:95. doi: 10.3389/fncir.2016.00095. eCollection 2016. Front Neural Circuits. 2016. PMID: 27932957 Free PMC article.
-
Multisensory temporal function and EEG complexity in patients with epilepsy and psychogenic nonepileptic events.Epilepsy Behav. 2017 May;70(Pt A):166-172. doi: 10.1016/j.yebeh.2017.02.018. Epub 2017 Apr 17. Epilepsy Behav. 2017. PMID: 28427027 Free PMC article.
-
Effects of developmental alcohol exposure on cortical multisensory integration.Eur J Neurosci. 2023 Mar;57(5):784-795. doi: 10.1111/ejn.15907. Epub 2023 Jan 24. Eur J Neurosci. 2023. PMID: 36610022 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

