Why are rods more sensitive than cones?
- PMID: 27218707
- PMCID: PMC5043029
- DOI: 10.1113/JP272556
Why are rods more sensitive than cones?
Abstract
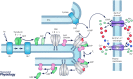
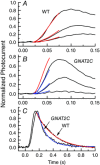
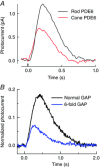
One hundred and fifty years ago Max Schultze first proposed the duplex theory of vision, that vertebrate eyes have two types of photoreceptor cells with differing sensitivity: rods for dim light and cones for bright light and colour detection. We now know that this division is fundamental not only to the photoreceptors themselves but to the whole of retinal and visual processing. But why are rods more sensitive, and how did the duplex retina first evolve? Cells resembling cones are very old, first appearing among cnidarians; the emergence of rods was a key step in the evolution of the vertebrate eye. Many transduction proteins have different isoforms in rods and cones, and others are expressed at different levels. Moreover rods and cones have a different anatomy, with only rods containing membranous discs enclosed by the plasma membrane. These differences must be responsible for the difference in absolute sensitivity, but which are essential? Recent research particularly expressing cone proteins in rods or changing the level of expression seem to show that many of the molecular differences in the activation and decay of the response may have each made a small contribution as evolution proceeded stepwise with incremental increases in sensitivity. Rod outer-segment discs were not essential and developed after single-photon detection. These experiments collectively provide a new understanding of the two kinds of photoreceptors and help to explain how gene duplication and the formation of rod-specific proteins produced the duplex retina, which has remained remarkably constant in physiology from amphibians to man.
Keywords: cones; photoreceptor; rhodopsin; rods; vision.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

