Hallmarks of cancer stem cell metabolism
- PMID: 27219018
- PMCID: PMC4984474
- DOI: 10.1038/bjc.2016.152
Hallmarks of cancer stem cell metabolism
Abstract
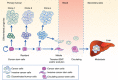
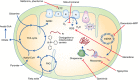
Cancer cells adapt cellular metabolism to cope with their high proliferation rate. Instead of primarily using oxidative phosphorylation (OXPHOS), cancer cells use less efficient glycolysis for the production of ATP and building blocks (Warburg effect). However, tumours are not uniform, but rather functionally heterogeneous and harbour a subset of cancer cells with stemness features. Such cancer cells have the ability to repopulate the entire tumour and thus have been termed cancer stem cells (CSCs) or tumour-initiating cells (TICs). As opposed to differentiated bulk tumour cells relying on glycolysis, CSCs show a distinct metabolic phenotype that, depending on the cancer type, can be highly glycolytic or OXPHOS dependent. In either case, mitochondrial function is critical and takes centre stage in CSC functionality. Remaining controversies in this young and emerging research field may be related to CSC isolation techniques and/or the use of less suitable model systems. Still, the apparent dependence of CSCs on mitochondrial function, regardless of their primary metabolic phenotype, represents a previously unrecognised Achilles heel amendable for therapeutic intervention. Elimination of highly chemoresistant CSCs as the root of many cancers via inhibition of mitochondrial function bears the potential to prevent relapse from disease and thus improve patients' long-term outcome.
Figures
References
-
- Carracedo A, Weiss D, Leliaert AK, Bhasin M, de Boer VC, Laurent G, Adams AC, Sundvall M, Song SJ, Ito K, Finley LS, Egia A, Libermann T, Gerhart-Hines Z, Puigserver P, Haigis MC, Maratos-Flier E, Richardson AL, Schafer ZT, Pandolfi PP (2012) A metabolic prosurvival role for PML in breast cancer. J Clin Invest 122(9): 3088–3100. - PMC - PubMed
-
- Chamberlain GR, Tulumello DV, Kelley SO (2013) Targeted delivery of doxorubicin to mitochondria. ACS Chem Biol 8(7): 1389–1395. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

