Global Aesthetics Consensus: Avoidance and Management of Complications from Hyaluronic Acid Fillers-Evidence- and Opinion-Based Review and Consensus Recommendations
- PMID: 27219265
- PMCID: PMC5242216
- DOI: 10.1097/PRS.0000000000002184
Global Aesthetics Consensus: Avoidance and Management of Complications from Hyaluronic Acid Fillers-Evidence- and Opinion-Based Review and Consensus Recommendations
Abstract
Background: Although the safety profile of hyaluronic acid fillers is favorable, adverse reactions can occur. Clinicians and patients can benefit from ongoing guidance on adverse reactions to hyaluronic acid fillers and their management.
Methods: A multinational, multidisciplinary group of experts in cosmetic medicine convened the Global Aesthetics Consensus Group to review the properties and clinical uses of Hylacross and Vycross hyaluronic acid products and develop updated consensus recommendations for early and late complications associated with hyaluronic acid fillers.
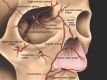
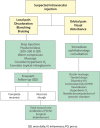
Results: The consensus panel provided specific recommendations focusing on early and late complications of hyaluronic acid fillers and their management. The impact of patient-, product-, and technique-related factors on such reactions was described. Most of these were noted to be mild and transient. Serious adverse events are rare. Early adverse reactions to hyaluronic acid fillers include vascular infarction and compromise; inflammatory reactions; injection-related events; and inappropriate placement of filler material. Among late reactions are nodules, granulomas, and skin discoloration. Most adverse events can be avoided with proper planning and technique. Detailed understanding of facial anatomy, proper patient and product selection, and appropriate technique can further reduce the risks. Should adverse reactions occur, the clinician must be prepared and have tools available for effective treatment.
Conclusions: Adverse reactions with hyaluronic acid fillers are uncommon. Clinicians should take steps to further reduce the risk and be prepared to treat any complications that arise.
Figures

References
-
- American Society for Aesthetic Plastic Surgery (ASAPS). 2014 Cosmetic Surgery National Data Bank statistics. Available at: http://www.surgery.org/sites/default/files/2014-Stats.pdf. Accessed June 21, 2015. - PubMed
-
- Alam M, Dover JS. Management of complications and sequelae with temporary injectable fillers. Plast Reconstr Surg. 2007;120(Suppl):98S–105S. - PubMed
-
- Friedman PM, Mafong EA, Kauvar AN, Geronemus RG. Safety data of injectable nonanimal stabilized hyaluronic acid gel for soft tissue augmentation. Dermatol Surg. 2002;28:491–494. - PubMed
-
- Narins RS, Jewell M, Rubin M, Cohen J, Strobos J. Clinical conference: Management of rare events following dermal fillers. Focal necrosis and angry red bumps. Dermatol Surg. 2006;32:426–434. - PubMed
-
- Ozturk CN, Li Y, Tung R, Parker L, Piliang MP, Zins JE. Complications following injection of soft-tissue fillers. Aesthet Surg J. 2013;33:862–877. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

