Cochlear perfusion with a viscous fluid
- PMID: 27220484
- PMCID: PMC4912859
- DOI: 10.1016/j.heares.2016.05.007
Cochlear perfusion with a viscous fluid
Abstract
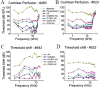
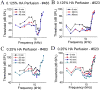
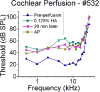
The flow of viscous fluid in the cochlea induces shear forces, which could provide benefit in clinical practice, for example to guide cochlear implant insertion or produce static pressure to the cochlear partition or wall. From a research standpoint, studying the effects of a viscous fluid in the cochlea provides data for better understanding cochlear fluid mechanics. However, cochlear perfusion with a viscous fluid may damage the cochlea. In this work we studied the physiological and anatomical effects of perfusing the cochlea with a viscous fluid. Gerbil cochleae were perfused at a rate of 2.4 μL/min with artificial perilymph (AP) and sodium hyaluronate (Healon, HA) in four different concentrations (0.0625%, 0.125%, 0.25%, 0.5%). The different HA concentrations were applied either sequentially in the same cochlea or individually in different cochleae. The perfusion fluid entered from the round window and was withdrawn from basal scala vestibuli, in order to perfuse the entire perilymphatic space. Compound action potentials (CAP) were measured after each perfusion. After perfusion with increasing concentrations of HA in the order of increasing viscosity, the CAP thresholds generally increased. The threshold elevation after AP and 0.0625% HA perfusion was small or almost zero, and the 0.125% HA was a borderline case, while the higher concentrations significantly elevated CAP thresholds. Histology of the cochleae perfused with the 0.0625% HA showed an intact Reissner's membrane (RM), while in cochleae perfused with 0.125% and 0.25% HA RM was torn. Thus, the CAP threshold elevation was likely due to the broken RM, likely caused by the shear stress produced by the flow of the viscous fluid. Our results and analysis indicate that the cochlea can sustain, without a significant CAP threshold shift, up to a 1.5 Pa shear stress. Beside these finding, in the 0.125% and 0.25% HA perfusion cases, a temporary CAP threshold shift was observed, perhaps due to the presence and then clearance of viscous fluid within the cochlea, or to a temporary position shift of the Organ of Corti. After 0.5% HA perfusion, a short latency positive peak (P0) appeared in the CAP waveform. This P0 might be due to a change in the cochlea's traveling-wave pattern, or distortion in the cochlear microphonic.
Keywords: Cochlear implantation; Cochlear mechanics; Cochlear perfusion; Compound action potential; Sodium hyaluronate; Viscous fluid.
Copyright © 2016 Elsevier B.V. All rights reserved.
Figures


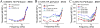



References
-
- Antoli-Candela F, Kiang NYS. Evoked Electrical Activity in the Auditory Nervous System. Academic Press; New York: 1978. Unit activity underlying the N1 potential; pp. 165–191.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

