The Fontan circulation after 45 years: update in physiology
- PMID: 27220691
- PMCID: PMC4941188
- DOI: 10.1136/heartjnl-2015-307467
The Fontan circulation after 45 years: update in physiology
Abstract
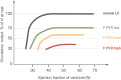
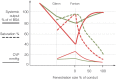
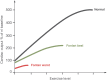
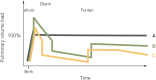
The Fontan operation was first performed in 1968. Since then, this operation has been performed on thousands of patients worldwide. Results vary from very good for many decades to very bad with a pleiad of complications and early death. A good understanding of the physiology is necessary to further improve results. The Fontan connection creates a critical bottleneck with obligatory upstream congestion and downstream decreased flow; these two features are the basic cause of the majority of the physiologic impairments of this circulation. The ventricle, while still the engine of the circuit, cannot compensate for the major flow restriction of the Fontan bottleneck: the suction required to compensate for the barrier effect cannot be generated, specifically not in a deprived heart. Except for some extreme situations, the heart therefore no longer controls cardiac output nor can it significantly alter the degree of systemic venous congestion. Adequate growth and development of the pulmonary arteries is extremely important as pulmonary vascular impedance will become the major determinant of Fontan outcome. Key features of the Fontan ventricle are early volume overload and overgrowth, but currently chronic preload deprivation with increasing filling pressures. A functional decline of the Fontan circuit is expected and observed as pulmonary vascular resistance and ventricular filling pressure increase with time. Treatment strategies will only be successful if they open up or bypass the critical bottleneck or act on immediate surroundings (impedance of the Fontan neoportal system, fenestration, enhanced ventricular suction).
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
