The Antenatal Corticosteroids Trial (ACT)'s explanations for neonatal mortality - a secondary analysis
- PMID: 27220987
- PMCID: PMC4878056
- DOI: 10.1186/s12978-016-0175-3
The Antenatal Corticosteroids Trial (ACT)'s explanations for neonatal mortality - a secondary analysis
Abstract
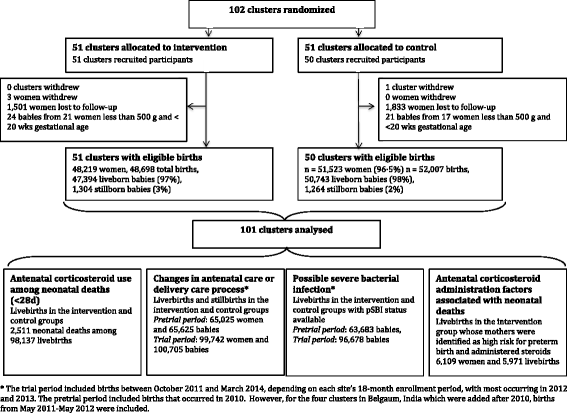
Background: The Antenatal Corticosteroid Trial assessed the feasibility, effectiveness, and safety of a multifaceted intervention to increase the use of antenatal corticosteroids (ACS) in mothers at risk of preterm birth at all levels of care in low and middle-income countries. The intervention effectively increased the use of ACS but was associated with an overall increase in neonatal deaths. We aimed to explore plausible pathways through which this intervention increased neonatal mortality.
Methods: We conducted a series of secondary analyses to assess whether ACS or other components of the multifaceted intervention that might have affected the quality of care contributed to the increased mortality observed: 1) we compared the proportion of neonatal deaths receiving ACS between the intervention and control groups; 2) we compared the antenatal and delivery care process in all births between groups; 3) we compared the rates of possible severe bacterial infection between groups; and 4) we compared the frequency of factors related to ACS administration or maternal high risk conditions at administration between the babies who died and those who survived 28 days among all births in the intervention group identified as high risk for preterm birth and received ACS.
Results: The ACS exposure among the infants who died up to 28 days was 29 % in the intervention group compared to 6 % in controls. No substantial differences were observed in antenatal and delivery care process between groups. The risk of pSBI plus neonatal death was significantly increased in intervention clusters compared to controls (2.4 % vs. 2.0 %, adjusted RR 1.17, 95 % CI 1.04-1.30, p = 0.008], primarily for infants with birth weight at or above the 25(th) percentile. Regarding factors related to ACS administration, term infants who died were more likely to have mothers who received ACS within 7 days of delivery compared to those who survived 28 days (26.5 % vs 17.9 %, p = 0.014), and their mothers were more likely to have been identified as high risk for hypertension and less likely for signs of preterm labor.
Conclusions: These results suggest that ACS more than other components of the intervention may have contributed to the overall increased neonatal mortality. ACS may have also been involved in the observed increased risk of neonatal infection and death. Further trials are urgently needed to clarify the effectiveness and safety of ACS on neonatal health in low resource settings.
References
-
- March of Dimes, PMNCH, Save the Children, WHO . In: Born Too Soon: The Global Action Report on Preterm Birth. Howson CP, Kinney MV, Lawn JE, editors. Geneva: World Health Organization; 2012.
-
- National Institutes of Health. The effect of corticosteroids for fetal maturation on perinatal outcomes. Consensus Development Conference Statement, Feb 28–March 2, 1994. http://consensus.nih.gov/1994/1994AntenatalSteroidPerinatal095html.htm. Accessed 1 June 2014.
-
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;3:1–141. - PubMed
-
- Katz J, Lee A, Kozuki N, the CHERG Small-for-Gestational-Age-Preterm Birth Working Group Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet. 2013;382:417–25. doi: 10.1016/S0140-6736(13)60993-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 HD058326/HD/NICHD NIH HHS/United States
- UG1 HD076461/HD/NICHD NIH HHS/United States
- K24 AT003683/AT/NCCIH NIH HHS/United States
- U01 HD058322/HD/NICHD NIH HHS/United States
- U10 HD078438/HD/NICHD NIH HHS/United States
- UG1 HD078439/HD/NICHD NIH HHS/United States
- U01 HD040607/HD/NICHD NIH HHS/United States
- U01 HD040657/HD/NICHD NIH HHS/United States
- U01 HD042372/HD/NICHD NIH HHS/United States
- U01 HD040636/HD/NICHD NIH HHS/United States
- U01 HD040477/HD/NICHD NIH HHS/United States
- U10 HD078437/HD/NICHD NIH HHS/United States
- U01 HD043464/HD/NICHD NIH HHS/United States
- U10 HD076461/HD/NICHD NIH HHS/United States
- U10 HD076457/HD/NICHD NIH HHS/United States
- UG1 HD076474/HD/NICHD NIH HHS/United States
- U10 HD076474/HD/NICHD NIH HHS/United States
- U10 HD078439/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical