Brodalumab for the Treatment of Psoriasis: A Review of Phase III Trials
- PMID: 27221323
- PMCID: PMC4906115
- DOI: 10.1007/s13555-016-0121-x
Brodalumab for the Treatment of Psoriasis: A Review of Phase III Trials
Abstract
Introduction: Interleukin (IL)-17 inhibitors are the most recent class of monoclonal antibodies approved by the FDA for psoriasis treatment. Preclinical and phase II studies of brodalumab, a high-affinity IL-17 receptor monoclonal antibody, have been encouraging.
Methods: We conducted a literature search using the PubMed database in order to assess the efficacy and safety profile of brodalumab. The search included the following key words: "psoriasis" and "IL-17" or "brodalumab." We also reviewed citations within articles to identify relevant sources.
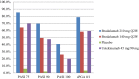
Results: At week 12, the proportion of patients attaining a 75% improvement from the baseline Psoriasis Area and Severity Index (PASI 75) was similar among the three phase III trials (AMAGINE-1, 83%; AMAGINE-2, 86%; AMAGINE-3, 85%). Brodalumab remained efficacious through 52 weeks of treatment. It maintained a satisfactory safety profile; the most frequently reported adverse events consisted of nasopharyngitis, headache, upper respiratory tract infection, and arthralgia.
Conclusion: Use of brodalumab revealed prompt clinical improvement and a favorable short-term safety profile in phase III trials, although further extension studies are needed to assess long-term safety. Based on the results, brodalumab appears to be a potent therapeutic option for patients with moderate-to-severe plaque-type psoriasis.
Keywords: AMAGINE; Anti-interleukin-17; Biologics; Brodalumab; Interleukin 17; Phase III; Psoriasis.
Figures
Similar articles
-
Brodalumab: the first anti-IL-17 receptor agent for psoriasis.Drugs Today (Barc). 2017 May;53(5):283-297. doi: 10.1358/dot.2017.53.5.2613690. Drugs Today (Barc). 2017. PMID: 28650001 Review.
-
Ixekizumab for the Treatment of Psoriasis: A Review of Phase III Trials.Dermatol Ther (Heidelb). 2016 Mar;6(1):25-37. doi: 10.1007/s13555-016-0102-0. Epub 2016 Feb 24. Dermatol Ther (Heidelb). 2016. PMID: 26910853 Free PMC article. Review.
-
Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: Efficacy and safety results from a phase II randomized controlled study.J Dermatol Sci. 2016 Jan;81(1):44-52. doi: 10.1016/j.jdermsci.2015.10.009. Epub 2015 Oct 24. J Dermatol Sci. 2016. PMID: 26547109 Clinical Trial.
-
Long-term efficacy and safety of brodalumab in moderate-to-severe plaque psoriasis: a post hoc pooled analysis of AMAGINE-2 and -3.J Eur Acad Dermatol Venereol. 2022 Aug;36(8):1275-1283. doi: 10.1111/jdv.18068. Epub 2022 Apr 1. J Eur Acad Dermatol Venereol. 2022. PMID: 35279890 Clinical Trial.
-
Impact of previous biologic use on the efficacy and safety of brodalumab and ustekinumab in patients with moderate-to-severe plaque psoriasis: integrated analysis of the randomized controlled trials AMAGINE-2 and AMAGINE-3.Br J Dermatol. 2018 Aug;179(2):320-328. doi: 10.1111/bjd.16464. Epub 2018 May 23. Br J Dermatol. 2018. PMID: 29488226 Clinical Trial.
Cited by
-
A Functional Variant rs3093023 in CCR6 Is Associated With IgA Nephropathy by Regulating Th17 Cells in a North Han Chinese Population.Front Immunol. 2021 Feb 25;12:600598. doi: 10.3389/fimmu.2021.600598. eCollection 2021. Front Immunol. 2021. PMID: 33717080 Free PMC article.
-
Brodalumab: First Global Approval.Drugs. 2016 Sep;76(14):1403-12. doi: 10.1007/s40265-016-0634-8. Drugs. 2016. PMID: 27577550
-
Circulating microRNA profile unveils mechanisms of action of acitretin for psoriasis vulgaris.Bioengineered. 2021 Dec;12(1):1838-1850. doi: 10.1080/21655979.2021.1925205. Bioengineered. 2021. PMID: 33975513 Free PMC article.
-
An Overview of Contemporary and Future Therapeutic Strategies for Scalp Psoriasis.Curr Drug Targets. 2024;25(5):353-373. doi: 10.2174/0113894501292755240304063020. Curr Drug Targets. 2024. PMID: 38500274 Review.
-
Therapeutic Potential of Targeting the Th17/Treg Axis in Autoimmune Disorders.Molecules. 2017 Jan 14;22(1):134. doi: 10.3390/molecules22010134. Molecules. 2017. PMID: 28098832 Free PMC article. Review.
References
-
- Rachakonda T, Schupp C, Armstrong A. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014 - PubMed
-
- Feldman SR, Malakouti M, Koo JY. Social impact of the burden of psoriasis: effects on patients and practice. Dermatol Online J. 2014;20(8):1–8. - PubMed
-
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70(5):871–81.e1–30. doi:10.1016/j.jaad.2013.12.018. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

