Luteolin alleviates methylglyoxal-induced cytotoxicity in osteoblastic MC3T3-E1 cells
- PMID: 27221336
- PMCID: PMC5101326
- DOI: 10.1007/s10616-016-9977-y
Luteolin alleviates methylglyoxal-induced cytotoxicity in osteoblastic MC3T3-E1 cells
Abstract
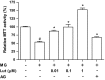
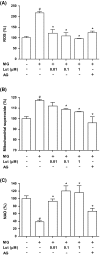
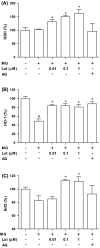
Methylglyoxal (MG), a reactive sugar-derived metabolite, exerts harmful effects by inducing oxidative stress, which aggravates a series of diabetic complications, including osteoporosis. The present study was performed to examine the effects of luteolin, a dietary polyphenolic flavonoid, on MG-induced cytotoxicity in MC3T3-E1 osteoblastic cells. Pretreatment of MC3T3-E1 osteoblastic cells with luteolin prevented MG-induced cell death and production of tumor necrosis factor-alpha, intracellular reactive oxygen species, mitochondrial superoxide, and cardiolipin peroxidation. In addition, luteolin increased the levels of glutathione and nuclear factor erythroid 2-related factor 2 (Nrf2) and decreased the inhibition of heme oxygenase-1 activity by MG. Pretreatment with luteolin prior to MG exposure reduced MG-induced mitochondrial dysfunction and increased the peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α) and nitric oxide levels, suggesting that luteolin may induce mitochondrial biogenesis. Taken together, these observations indicated that luteolin has potential as a preventive agent against the development of diabetic osteopathy related to MG-induced oxidative stress in diabetes.
Keywords: Glutathione; Mitochondrial function; Nitric oxide; Osteoblasts; Reactive oxygen species.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


Similar articles
-
Protective effect of liquiritigenin against methylglyoxal cytotoxicity in osteoblastic MC3T3-E1 cells.Food Funct. 2014 Jul 25;5(7):1432-40. doi: 10.1039/c4fo00127c. Food Funct. 2014. PMID: 24789098
-
Sciadopitysin alleviates methylglyoxal-mediated glycation in osteoblastic MC3T3-E1 cells by enhancing glyoxalase system and mitochondrial biogenesis.Free Radic Res. 2014 Jul;48(7):729-39. doi: 10.3109/10715762.2014.903562. Epub 2014 Apr 3. Free Radic Res. 2014. PMID: 24628445
-
Glabridin Alleviates the Toxic Effects of Methylglyoxal on Osteoblastic MC3T3-E1 Cells by Increasing Expression of the Glyoxalase System and Nrf2/HO-1 Signaling and Protecting Mitochondrial Function.J Agric Food Chem. 2016 Jan 13;64(1):226-35. doi: 10.1021/acs.jafc.5b05157. Epub 2016 Jan 5. J Agric Food Chem. 2016. PMID: 26670935
-
Actein protects against methylglyoxal-induced oxidative damage in osteoblastic MC3T3-E1 cells.J Sci Food Agric. 2017 Jan;97(1):207-214. doi: 10.1002/jsfa.7713. Epub 2016 Apr 15. J Sci Food Agric. 2017. PMID: 26991449
-
Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders.Neurol Res. 2017 Jan;39(1):73-82. doi: 10.1080/01616412.2016.1251711. Epub 2016 Nov 3. Neurol Res. 2017. PMID: 27809706 Review.
Cited by
-
The Role of NRF2 in Bone Metabolism - Friend or Foe?Front Endocrinol (Lausanne). 2022 Feb 23;13:813057. doi: 10.3389/fendo.2022.813057. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35282459 Free PMC article. Review.
-
[FER-1 inhibits methylglyoxal-induced ferroptosis in mouse alveolar macrophages in vitro].Nan Fang Yi Ke Da Xue Xue Bao. 2024 Dec 20;44(12):2443-2448. doi: 10.12122/j.issn.1673-4254.2024.12.21. Nan Fang Yi Ke Da Xue Xue Bao. 2024. PMID: 39725634 Free PMC article. Chinese.
-
[Role of interaction between reactive oxygen species and ferroptosis pathway in methylglyoxal-induced injury in mouse embryonic osteoblasts].Nan Fang Yi Ke Da Xue Xue Bao. 2022 Jan 20;42(1):108-115. doi: 10.12122/j.issn.1673-4254.2022.01.13. Nan Fang Yi Ke Da Xue Xue Bao. 2022. PMID: 35249877 Free PMC article. Chinese.
-
Luteolin supports osteogenic differentiation of human periodontal ligament cells.BMC Oral Health. 2019 Oct 26;19(1):229. doi: 10.1186/s12903-019-0926-y. BMC Oral Health. 2019. PMID: 31655580 Free PMC article.
-
Tamarind: A diet-based strategy against lifestyle maladies.Food Sci Nutr. 2019 Sep 27;7(11):3378-3390. doi: 10.1002/fsn3.1218. eCollection 2019 Nov. Food Sci Nutr. 2019. Retraction in: Food Sci Nutr. 2024 Apr 05;12(5):3775. doi: 10.1002/fsn3.4091. PMID: 31762991 Free PMC article. Retracted. Review.
References
-
- Addabbo F, Ratliff B, Park HC, Kuo M, Ungvari Z, Csiszar A, Krasnikov B, Sodhi K, Zhang F, Nasjletti A, Goligorsky MS. The Krebs cycle and mitochondrial mass are early victims of endothelial dysfunction: proteomic approach. Am J Pathol. 2009;174:34–43. doi: 10.2353/ajpath.2009.080650. - DOI - PMC - PubMed
-
- Arredondo F, Echeverry C, Abin-Carriquiry JA, Blasina F, Antunez K, Jones DP, Go YM, Liang YL, Dajas F. After cellular internalization, quercetin causes Nrf2 nuclear translocation, increases glutathione levels, and prevents neuronal death against an oxidative insult. Free Radic Biol Med. 2010;49:738–747. doi: 10.1016/j.freeradbiomed.2010.05.020. - DOI - PubMed
-
- Blatnik M, Frizzell N, Thorpe SR, Baynes JW. Inactivation of glyceraldehyde-3-phosphate dehydrogenase by fumarate in diabetes: formation of S-(2-succinyl)cysteine, a novel chemical modification of protein and possible biomarker of mitochondrial stress. Diabetes. 2008;57:41–49. doi: 10.2337/db07-0838. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

