Origin and spread of HIV-1 in persons who inject drugs in Bulgaria
- PMID: 27221346
- PMCID: PMC11318570
- DOI: 10.1016/j.meegid.2016.05.029
Origin and spread of HIV-1 in persons who inject drugs in Bulgaria
Abstract
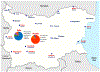
Increased HIV transmission in persons who inject drugs (PWIDs) has led to subepidemics and outbreaks in several countries in Europe, including Bulgaria. In this study in Bulgaria, we investigate the origin and spatiotemporal evolutionary history of HIV-1 infections in PWIDs and the distribution of antiretroviral resistance mutations and hepatitis co-infections in these populations. We analyzed HIV-1 polymerase sequences available from 117 of 359 PWIDs diagnosed with HIV/AIDS from 1999 to 2011. Of these, 50 (42.7%) were classified as CRF02_AG, 41 (35.0%) CRF01_AE, 12 (10.3%) URFs, ten (8.5%) subtype B, two (1.7%) subtype F1 and two (1.7%) CRF14_BG. Most recent common ancestor dating suggests that CRF01_AE was likely first introduced from Southeast Asia into persons reporting heterosexual infection in Bulgaria in 1992 and spread subsequently to PWIDs in the capital city of Sofia around 2003. Conversely, CRF02_AG in Bulgaria was likely first introduced into PWID from Germany in 2000 and later entered heterosexual populations around 2009. The overall prevalence of resistance mutations was 6.8% (8/117), of which 5.1% (5/117) was observed in patients on antiretroviral therapy and 1.7% (2/117) was from transmitted drug resistance mutations in drug-naïve individuals. 189/204 (92.6%) PWIDs were also co-infected with hepatitis C (HCV) and 31/183 (16.9%) were co-infected with hepatitis B (HBV). Our study provides valuable molecular epidemiological information on the introduction and distribution of the main HIV-1 subtypes, resistance mutations and hepatitis co-infections among PWIDs with HIV-1 in Bulgaria which can be used to target prevention efforts.
Keywords: Drug resistance; HIV; Hepatitis; Injection drug use; Molecular epidemiology; Subtype.
Copyright © 2016 Elsevier B.V. All rights reserved.
Conflict of interest statement
Transparency declaration
The authors declare no conflicts of interest.
Figures




References
-
- Aceijas C, Rhodes T, 2007. Global estimates of prevalence of HCV infection among injecting drug users. Int. J. Drug Policy 18, 352–358. - PubMed
-
- Alexiev I, Beshkov D, Shankar A, Hanson D, Paraskevis D, Georgieva V, Karamacheva L , Taskov H, Varleva T, Elenkov I, Stoicheva M, Nikolova D, Switzer W, 2013. Detailed molecular epidemiologic characterization of HIV-1 infection in Bulgaria reveals broad diversity and evolving phylodynamics. PloS one 8, e59666. - PMC - PubMed
-
- Angelis K, Albert J, Mamais I, Magiorkinis G, Hatzakis A, Hamouda O, Struck D, Vercauteren J, Wensing AM, Alexiev I, Asjo B, Balotta C, Camacho RJ, Coughlan S, Griskevicius A, Z. G, Horban A, Kostrikis LG, Lepej S, Liitsola K, Linka M, Nielsen C, Otelea D, Paredes R, Poljak M, Puchhammer-Stockl E, Schmit JC, Sonnerborg A, Stanekova D, Stanojevic M, Boucher CA, Kaplan L, Vandamme AM, Paraskevis D, 2015. Global dispersal pattern of HIV type 1 subtype CRF01_AE: a genetic trace of human mobility related to heterosexual sexual activities centralized in Southeast Asia. J. Infect. Dis 211, 1735–1744. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

