The endometrial preparation for frozen-thawed euploid blastocyst transfer: a prospective randomized trial comparing clinical results from natural modified cycle and exogenous hormone stimulation with GnRH agonist
- PMID: 27221477
- PMCID: PMC4930788
- DOI: 10.1007/s10815-016-0736-y
The endometrial preparation for frozen-thawed euploid blastocyst transfer: a prospective randomized trial comparing clinical results from natural modified cycle and exogenous hormone stimulation with GnRH agonist
Abstract
Purpose: The aim of the study was to evaluate two methods of endometrial preparation for frozen-thawed single euploid blastocyst transfer: modified natural and artificial cycle with GnRH-agonist pituitary suppression.
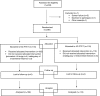
Methods: In this prospective, controlled randomized trial, a total of 236 patients undergoing infertility treatment were randomized in 1:1 ratio; 118 received a frozen-thawed single euploid blastocyst transfer in a modified natural cycle and 118 in an artificial cycle with GnRH-agonist pituitary suppression. In the artificial protocol, GnRH-agonist combined with estradiol valerate was administered. In the natural protocol, only final oocyte maturation was induced using human chorionic gonadotropin administration. The primary end-points were the clinical pregnancy and implantation rates; the secondary end-points were the cost-benefit in terms of drug cost and the number of visits and the woman psychological distress caused by the treatment.
Results: No significant differences were found in clinical pregnancy, implantation, and miscarriage rates between protocols. The number of clinical and ultrasound controls and the number of laboratory dosages and venous samplings were similar in both study groups. No significant differences were found between the groups in the anxiety and depression values before the start of treatment, on the days of progesterone administration, the blastocyst transfer, and pregnancy test.
Conclusions: The findings of this study evidence that in case of frozen-thawed single euploid blastocyst transfer, both protocols are equally effective in terms of clinical outcomes, cost-benefit, and patient compliance. The choice of endometrial preparation protocol should be based on women menstrual and ovulatory characteristics or otherwise on patient need for cycle planning.
Trial registration: www.clinicaltrials.gov with number NCT02378584.
Keywords: Blastocyst transfer; Endometrial preparation; Euploid embryos; Vitrification.
References
-
- Hill MJ, Miller A, Frattarelli JL. A GnRH agonist and exogenous hormone stimulation protocol has a higher live-birth rate than a natural endogenous hormone protocol for frozen-thawed blastocyst-stage embryo transfer cycles: an analysis of 1391 cycles. Fertil Steril. 2010;93:416–422. doi: 10.1016/j.fertnstert.2008.11.027. - DOI - PubMed
-
- Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril. 2011;96:344–348. doi: 10.1016/j.fertnstert.2011.05.050. - DOI - PubMed
-
- Munné S, Lee A, Rosenwaks Z, Grifo J, Cohen J. Diagnosis of major chromosome aneuploidies in human preimplantation embryos. Hum Reprod. 1993;8(12):2185–2191. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical