The synthesis, regulation, and functions of sterols in Candida albicans: Well-known but still lots to learn
- PMID: 27221657
- PMCID: PMC4991322
- DOI: 10.1080/21505594.2016.1188236
The synthesis, regulation, and functions of sterols in Candida albicans: Well-known but still lots to learn
Abstract
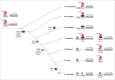
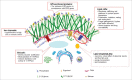
Sterols are the basal components of the membranes of the fungal pathogen Candida albicans, and these membranes determine the susceptibility of C. albicans cells to a variety of stresses, such as ionic, osmotic and oxidative pressures, and treatment with antifungal drugs. The common antifungal azoles in clinical use are targeted to the biosynthesis of ergosterol. In the past years, the synthesis, storage and metabolism of ergosterol in Saccharomyces cerevisiae has been characterized in some detail; however, these processes has not been as well investigated in the human opportunistic pathogen C. albicans. In this review, we summarize the genes involved in ergosterol synthesis and regulation in C. albicans. As well, genes in S. cerevisiae implicated in ergosterol storage and conversions with other lipids are noted, as these provide us clues and directions for the study of the homologous genes in C. albicans. In this report we have particularly focused on the essential roles of ergosterol in the dynamic process of cell biology and its fundamental status in the biological membrane system that includes lipid rafts, lipid droplets, vacuoles and mitochondria. We believe that a thorough understanding of this classic and essential pathway will give us new ideas about drug resistance and morphological switching in C. albicans.
Keywords: Candida albicans; biosynthesis; function; regulation; sterols.
Figures

Similar articles
-
Candida albicans zinc cluster protein Upc2p confers resistance to antifungal drugs and is an activator of ergosterol biosynthetic genes.Antimicrob Agents Chemother. 2005 May;49(5):1745-52. doi: 10.1128/AAC.49.5.1745-1752.2005. Antimicrob Agents Chemother. 2005. PMID: 15855491 Free PMC article.
-
Mitochondria influence CDR1 efflux pump activity, Hog1-mediated oxidative stress pathway, iron homeostasis, and ergosterol levels in Candida albicans.Antimicrob Agents Chemother. 2013 Nov;57(11):5580-99. doi: 10.1128/AAC.00889-13. Epub 2013 Aug 26. Antimicrob Agents Chemother. 2013. PMID: 23979757 Free PMC article.
-
Role of Candida albicans transcription factor Upc2p in drug resistance and sterol metabolism.Eukaryot Cell. 2004 Dec;3(6):1391-7. doi: 10.1128/EC.3.6.1391-1397.2004. Eukaryot Cell. 2004. PMID: 15590814 Free PMC article.
-
Beyond ergosterol: linking pH to antifungal mechanisms.Virulence. 2010 Nov-Dec;1(6):551-4. doi: 10.4161/viru.1.6.13802. Epub 2010 Nov 1. Virulence. 2010. PMID: 21178501 Review.
-
Regulation of Ergosterol Biosynthesis in Saccharomyces cerevisiae.Genes (Basel). 2020 Jul 15;11(7):795. doi: 10.3390/genes11070795. Genes (Basel). 2020. PMID: 32679672 Free PMC article. Review.
Cited by
-
Carvacrol-Induced Vacuole Dysfunction and Morphological Consequences in Nakaseomyces glabratus and Candida albicans.Microorganisms. 2023 Dec 4;11(12):2915. doi: 10.3390/microorganisms11122915. Microorganisms. 2023. PMID: 38138059 Free PMC article.
-
Staphylococcus aureus Synergized with Candida albicans to Increase the Pathogenesis and Drug Resistance in Cutaneous Abscess and Peritonitis Murine Models.Pathogens. 2021 Aug 16;10(8):1036. doi: 10.3390/pathogens10081036. Pathogens. 2021. PMID: 34451500 Free PMC article.
-
In silico molecular modelling studies and antibiofilm efficacy of shikonin against Candida albicans: mechanistic insight.Arch Microbiol. 2023 Feb 17;205(3):93. doi: 10.1007/s00203-023-03426-x. Arch Microbiol. 2023. PMID: 36800037
-
Alterations in the Level of Ergosterol in Candida albicans' Plasma Membrane Correspond with Changes in Virulence and Result in Triggering Diversed Inflammatory Response.Int J Mol Sci. 2023 Feb 16;24(4):3966. doi: 10.3390/ijms24043966. Int J Mol Sci. 2023. PMID: 36835379 Free PMC article.
-
Molecular investigations on Candida glabrata clinical isolates for pharmacological targeting.RSC Adv. 2022 Jun 14;12(27):17570-17584. doi: 10.1039/d2ra02092k. eCollection 2022 Jun 7. RSC Adv. 2022. PMID: 35765448 Free PMC article.
References
-
- Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med 2012; 4:165rv13; PMID:23253612 - PubMed
-
- Martins N, Ferreira IC, Barros L, Silva S, Henriques M. Candidiasis: predisposing factors, prevention, diagnosis and alternative treatment. Mycopathologia 2014; 177:223-40; PMID:24789109; http://dx.doi.org/10.1007/s11046-014-9749-1 - DOI - PubMed
-
- Parker JE, Warrilow AG, Price CL, Mullins JG, Kelly DE, Kelly SL. Resistance to antifungals that target CYP51. J Chem Biol 2014; 7:143-61; PMID:25320648; http://dx.doi.org/10.1007/s12154-014-0121-1 - DOI - PMC - PubMed
-
- Ostrosky-Zeichner L, Casadevall A, Galgiani JN, Odds FC, Rex JH. An insight into the antifungal pipeline: selected new molecules and beyond. Nat Rev Drug Discov 2010; 9:719-27; PMID:20725094; http://dx.doi.org/10.1038/nrd3074 - DOI - PubMed
-
- Solomon SL, Oliver KB. Antibiotic resistance threats in the United States: stepping back from the brink. Am Fam Physician 2014; 89:938-41; PMID:25162160 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
