Prediction of rates of thromboembolic and major bleeding outcomes with dabigatran or warfarin among patients with atrial fibrillation: new initiator cohort study
- PMID: 27221664
- PMCID: PMC4878389
- DOI: 10.1136/bmj.i2607
Prediction of rates of thromboembolic and major bleeding outcomes with dabigatran or warfarin among patients with atrial fibrillation: new initiator cohort study
Abstract
Objectives: To compare stratified event rates from randomized controlled trials with predicted event rates from models developed in observational data, and assess their ability to accurately capture observed rates of thromboembolism and major bleeding for patients treated with dabigatran or warfarin as part of routine care.
Design: New initiator cohort study.
Setting: Data from United Health (October 2009 to June 2013), a commercial healthcare claims database in the United States.
Participants: 21 934 adults with atrial fibrillation initiating dabigatran (150 mg dose only) or warfarin treatment as part of routine care.
Main outcome measures: Predicted annual rates of thromboembolism or major bleeding, based on estimates from randomized controlled trials, models developed in routine care patients, and baseline risk scores (CHADS2, CHA2DS2-VASc, and HAS-BLED). Thromboembolism was a composite outcome, including primary inpatient diagnosis codes for ischemic or ill defined stroke, transient ischemic attack, pulmonary embolism, deep vein thrombosis, and systemic embolism. Major bleeding was a composite outcome including codes occurring in an inpatient setting for hemorrhagic stroke; major upper, lower, or unspecified gastrointestinal bleed; and major urogenital or other bleed.
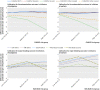
Results: 6516 (30%) and 15 418 (70%) of patients initiated dabigatran and warfarin, respectively. Annual event rates per 100 patients were 1.7 for thromboembolism and 4.6 for major bleeding. For thromboembolism, calibration of estimates from randomized controlled trials was similar to calibration for model based predictions; however, trial estimates for major bleeding consistently underestimated the rate of bleeding among patients in routine care. Underestimation of bleeding rates was particularly pronounced in warfarin initiators with high HAS-BLED scores, where event rates were underestimated by up to 4.0 per 100 patient years. Harrell's c indices for discrimination for thromboembolism or major bleeding in dabigatran and warfarin initiators ranged between 0.59 and 0.66 for randomized controlled trial predictions, and between 0.52 and 0.70 for cross validated model based predictions.
Conclusion: Estimated rates of thromboembolism under dabigatran or warfarin treatment in randomized controlled trials were close to observed rates in routine care patients. However, rates of major bleeding were underestimated. Models developed in routine care patients can provide accurate, tailored estimates of risk and benefit under alternative treatment to enhance patient centered care.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures

References
-
- Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263-72. 10.1378/chest.09-1584 pmid:19762550. - DOI - PubMed
-
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093-100. 10.1378/chest.10-0134 pmid:20299623. - DOI - PubMed
-
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51. 10.1056/NEJMoa0905561 pmid:19717844. - DOI - PubMed
-
- Granger CB, Alexander JH, McMurray JJV, et al. ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92. 10.1056/NEJMoa1107039 pmid:21870978. - DOI - PubMed
-
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857-67. 10.7326/0003-4819-146-12-200706190-00007 pmid:17577005. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
