Leptin directly promotes T-cell glycolytic metabolism to drive effector T-cell differentiation in a mouse model of autoimmunity
- PMID: 27222115
- PMCID: PMC5154618
- DOI: 10.1002/eji.201545861
Leptin directly promotes T-cell glycolytic metabolism to drive effector T-cell differentiation in a mouse model of autoimmunity
Abstract
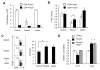
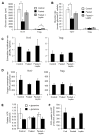
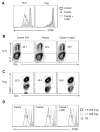
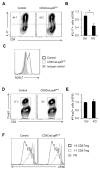
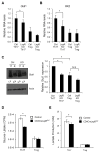
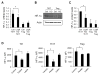
Upon activation, T cells require energy for growth, proliferation, and function. Effector T (Teff) cells, such as Th1 and Th17 cells, utilize high levels of glycolytic metabolism to fuel proliferation and function. In contrast, Treg cells require oxidative metabolism to fuel suppressive function. It remains unknown how Teff/Treg-cell metabolism is altered when nutrients are limited and leptin levels are low. We therefore examined the role of malnutrition and associated hypoleptinemia on Teff versus Treg cells. We found that both malnutrition-associated hypoleptinemia and T cell-specific leptin receptor knockout suppressed Teff-cell number, function, and glucose metabolism, but did not alter Treg-cell metabolism or suppressive function. Using the autoimmune mouse model EAE, we confirmed that fasting-induced hypoleptinemia altered Teff-cell, but not Treg-cell, glucose metabolism, and function in vivo, leading to decreased disease severity. To explore potential mechanisms, we examined HIF-1α, a key regulator of Th17 differentiation and Teff-cell glucose metabolism, and found HIF-1α expression was decreased in T cell-specific leptin receptor knockout Th17 cells, and in Teff cells from fasted EAE mice, but was unchanged in Treg cells. Altogether, these data demonstrate a selective, cell-intrinsic requirement for leptin to upregulate glucose metabolism and maintain function in Teff, but not Treg cells.
Keywords: EAE; Glucose metabolism; HIF-1α; Leptin; Malnutrition; Th17; Treg cells.
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Statement The authors declare no commercial or financial conflict of interest.
Figures

References
-
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. - PubMed
-
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. - PubMed
-
- Cosmi L, Liotta F, Maggi E, Romagnani S, Annunziato F. Th17 and non-classic Th1 cells in chronic inflammatory disorders: two sides of the same coin. Int Arch Allergy Immunol. 2014;164:171–177. - PubMed
-
- Eisenstein EM, Williams CB. The T(reg)/Th17 cell balance: a new paradigm for autoimmunity. Pediatr Res. 2009;65:26R–31R. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

