A ventral root avulsion injury model for neurogenic underactive bladder studies
- PMID: 27222131
- PMCID: PMC5077668
- DOI: 10.1016/j.expneurol.2016.05.026
A ventral root avulsion injury model for neurogenic underactive bladder studies
Abstract
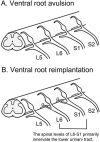
Detrusor underactivity (DU) is defined as a contraction of reduced strength and/or duration during bladder emptying and results in incomplete and prolonged bladder emptying. The clinical diagnosis of DU is challenging when present alone or in association with other bladder conditions such as detrusor overactivity, urinary retention, detrusor hyperactivity with impaired contractility, aging, and neurological injuries. Several etiologies may be responsible for DU or the development of an underactive bladder (UAB), but the pathobiology of DU or UAB is not well understood. Therefore, new clinically relevant and interpretable models for studies of UAB are much needed in order to make progress towards new treatments and preventative strategies. Here, we review a neuropathic cause of DU in the form of traumatic injuries to the cauda equina (CE) and conus medullaris (CM) portions of the spinal cord. Lumbosacral ventral root avulsion (VRA) injury models in rats mimic the clinical phenotype of CM/CE injuries. Bilateral VRA injuries result in bladder areflexia, whereas a unilateral lesion results in partial impairment of lower urinary tract and visceromotor reflexes. Surgical re-implantation of avulsed ventral roots into the spinal cord and pharmacological strategies can augment micturition reflexes. The translational research need for the development of a large animal model for UAB studies is also presented, and early studies of lumbosacral VRA injuries in rhesus macaques are discussed.
Keywords: And conus medullaris; Cauda equine; Electromyography; External urethral sphincter; Serotonin; Spinal cord injury.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

References
-
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, Van Kerrebroeck P, Victor A, Wein A, Standardisation Sub-Committee of the International Continence, S. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37–49. - PubMed
-
- Benarroch EE. CGRP: sensory neuropeptide with multiple neurologic implications. Neurology. 2011;77:281–287. - PubMed
-
- Beric A, Light JK. Function of the conus medullaris and cauda equina in the early period following spinal cord injury and the relationship to recovery of detrusor function. J Urol. 1992;148:1845–1848. - PubMed
-
- Carlstedt T, Anand P, Hallin R, Misra PV, Noren G, Seferlis T. Spinal nerve root repair and reimplantation of avulsed ventral roots into the spinal cord after brachial plexus injury. J Neurosurg. 2000;93:237–247. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

