Inhibition of Rac1 reduces store overload-induced calcium release and protects against ventricular arrhythmia
- PMID: 27222313
- PMCID: PMC4956946
- DOI: 10.1111/jcmm.12840
Inhibition of Rac1 reduces store overload-induced calcium release and protects against ventricular arrhythmia
Abstract
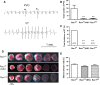
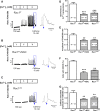
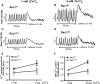
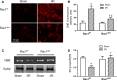
Rac1 is a small GTPase and plays key roles in multiple cellular processes including the production of reactive oxygen species (ROS). However, whether Rac1 activation during myocardial ischaemia and reperfusion (I/R) contributes to arrhythmogenesis is not fully understood. We aimed to study the effects of Rac1 inhibition on store overload-induced Ca(2+) release (SOICR) and ventricular arrhythmia during myocardial I/R. Adult Rac1(f/f) and cardiac-specific Rac1 knockdown (Rac1(ckd) ) mice were subjected to myocardial I/R and their electrocardiograms (ECGs) were monitored for ventricular arrhythmia. Myocardial Rac1 activity was increased and ventricular arrhythmia was induced during I/R in Rac1(f/f) mice. Remarkably, I/R-induced ventricular arrhythmia was significantly decreased in Rac1(ckd) compared to Rac1(f/f) mice. Furthermore, treatment with Rac1 inhibitor NSC23766 decreased I/R-induced ventricular arrhythmia. Ca(2+) imaging analysis showed that in response to a 6 mM external Ca(2+) concentration challenge, SOICR was induced with characteristic spontaneous intracellular Ca(2+) waves in Rac1(f/f) cardiomyocytes. Notably, SOICR was diminished by pharmacological and genetic inhibition of Rac1 in adult cardiomyocytes. Moreover, I/R-induced ROS production and ryanodine receptor 2 (RyR2) oxidation were significantly inhibited in the myocardium of Rac1(ckd) mice. We conclude that Rac1 activation induces ventricular arrhythmia during myocardial I/R. Inhibition of Rac1 suppresses SOICR and protects against ventricular arrhythmia. Blockade of Rac1 activation may represent a new paradigm for the treatment of cardiac arrhythmia in ischaemic heart disease.
Keywords: Rac1; arrhythmia; ischaemia and reperfusion; reactive oxygen species; store overload-induced calcium release.
© 2016 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures

Similar articles
-
The arrhythmogenic human HRC point mutation S96A leads to spontaneous Ca(2+) release due to an impaired ability to buffer store Ca(2+).J Mol Cell Cardiol. 2014 Sep;74:22-31. doi: 10.1016/j.yjmcc.2014.04.019. Epub 2014 May 5. J Mol Cell Cardiol. 2014. PMID: 24805197
-
Nebivolol suppresses cardiac ryanodine receptor-mediated spontaneous Ca2+ release and catecholaminergic polymorphic ventricular tachycardia.Biochem J. 2016 Nov 15;473(22):4159-4172. doi: 10.1042/BCJ20160620. Epub 2016 Sep 13. Biochem J. 2016. PMID: 27623776 Free PMC article.
-
Channel Activity of Cardiac Ryanodine Receptors (RyR2) Determines Potency and Efficacy of Flecainide and R-Propafenone against Arrhythmogenic Calcium Waves in Ventricular Cardiomyocytes.PLoS One. 2015 Jun 29;10(6):e0131179. doi: 10.1371/journal.pone.0131179. eCollection 2015. PLoS One. 2015. PMID: 26121139 Free PMC article.
-
[Molecular mechanism of defective intracellular calcium release in heart failure and lethal arrhythmia].Nihon Yakurigaku Zasshi. 2012 Dec;140(6):250-4. doi: 10.1254/fpj.140.250. Nihon Yakurigaku Zasshi. 2012. PMID: 23229629 Review. Japanese. No abstract available.
-
Mechanisms and potential therapeutic targets for ventricular arrhythmias associated with impaired cardiac calcium cycling.J Mol Cell Cardiol. 2008 Jan;44(1):31-43. doi: 10.1016/j.yjmcc.2007.10.012. Epub 2007 Oct 25. J Mol Cell Cardiol. 2008. PMID: 18061204 Free PMC article. Review.
Cited by
-
Nexinhib20 Inhibits Neutrophil Adhesion and β2 Integrin Activation by Antagonizing Rac-1-Guanosine 5'-Triphosphate Interaction.J Immunol. 2022 Oct 15;209(8):1574-1585. doi: 10.4049/jimmunol.2101112. Epub 2022 Sep 7. J Immunol. 2022. PMID: 36165184 Free PMC article.
-
RAC1 is involved in uterine myometrium contraction in the inflammation-associated preterm birth.Reproduction. 2022 Sep 20;164(4):169-181. doi: 10.1530/REP-21-0186. Print 2022 Oct 1. Reproduction. 2022. PMID: 36018772 Free PMC article.
-
Inhibition of GTPase Rac1 expression by vitamin D mitigates pressure overload-induced cardiac hypertrophy.Int J Cardiol Heart Vasc. 2021 Nov 30;37:100922. doi: 10.1016/j.ijcha.2021.100922. eCollection 2021 Dec. Int J Cardiol Heart Vasc. 2021. PMID: 34917751 Free PMC article.
-
Ischemia-reperfusion injury: molecular mechanisms and therapeutic targets.Signal Transduct Target Ther. 2024 Jan 8;9(1):12. doi: 10.1038/s41392-023-01688-x. Signal Transduct Target Ther. 2024. PMID: 38185705 Free PMC article. Review.
-
8-Aminoguanine Induces Diuresis, Natriuresis, and Glucosuria by Inhibiting Purine Nucleoside Phosphorylase and Reduces Potassium Excretion by Inhibiting Rac1.J Am Heart Assoc. 2018 Nov 6;7(21):e010085. doi: 10.1161/JAHA.118.010085. J Am Heart Assoc. 2018. PMID: 30608204 Free PMC article.
References
-
- Verma S, Fedak PW, Weisel RD, et al Fundamentals of reperfusion injury for the clinical cardiologist. Circulation. 2002; 105: 2332–6. - PubMed
-
- Sanada S, Komuro I, Kitakaze M. Pathophysiology of myocardial reperfusion injury: preconditioning, postconditioning, and translational aspects of protective measures. Am J Physiol Heart Circ Physiol. 2011; 301: H1723–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

