Staphylococcus aureus Protein A Mediates Interspecies Interactions at the Cell Surface of Pseudomonas aeruginosa
- PMID: 27222468
- PMCID: PMC4895107
- DOI: 10.1128/mBio.00538-16
Staphylococcus aureus Protein A Mediates Interspecies Interactions at the Cell Surface of Pseudomonas aeruginosa
Abstract
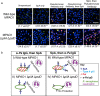
While considerable research has focused on the properties of individual bacteria, relatively little is known about how microbial interspecies interactions alter bacterial behaviors and pathogenesis. Staphylococcus aureus frequently coinfects with other pathogens in a range of different infectious diseases. For example, coinfection by S. aureus with Pseudomonas aeruginosa occurs commonly in people with cystic fibrosis and is associated with higher lung disease morbidity and mortality. S. aureus secretes numerous exoproducts that are known to interact with host tissues, influencing inflammatory responses. The abundantly secreted S. aureus staphylococcal protein A (SpA) binds a range of human glycoproteins, immunoglobulins, and other molecules, with diverse effects on the host, including inhibition of phagocytosis of S. aureus cells. However, the potential effects of SpA and other S. aureus exoproducts on coinfecting bacteria have not been explored. Here, we show that S. aureus-secreted products, including SpA, significantly alter two behaviors associated with persistent infection. We found that SpA inhibited biofilm formation by specific P. aeruginosa clinical isolates, and it also inhibited phagocytosis by neutrophils of all isolates tested. Our results indicate that these effects were mediated by binding to at least two P. aeruginosa cell surface structures-type IV pili and the exopolysaccharide Psl-that confer attachment to surfaces and to other bacterial cells. Thus, we found that the role of a well-studied S. aureus exoproduct, SpA, extends well beyond interactions with the host immune system. Secreted SpA alters multiple persistence-associated behaviors of another common microbial community member, likely influencing cocolonization and coinfection with other microbes.
Importance: Bacteria rarely exist in isolation, whether on human tissues or in the environment, and they frequently coinfect with other microbes. However, relatively little is known about how microbial interspecies interactions alter bacterial behaviors and pathogenesis. We identified a novel interaction between two bacterial species that frequently infect together-Staphylococcus aureus and Pseudomonas aeruginosa We show that the S. aureus-secreted protein staphylococcal protein A (SpA), which is well-known for interacting with host targets, also binds to specific P. aeruginosa cell surface molecules and alters two persistence-associated P. aeruginosa behaviors: biofilm formation and uptake by host immune cells. Because S. aureus frequently precedes P. aeruginosa in chronic infections, these findings reveal how microbial community interactions can impact persistence and host interactions during coinfections.
Copyright © 2016 Armbruster et al.
Figures
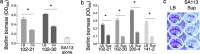
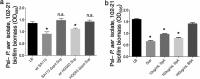
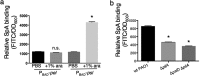
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
