Antagonism of the Sodium-Potassium ATPase Impairs Chikungunya Virus Infection
- PMID: 27222471
- PMCID: PMC4895112
- DOI: 10.1128/mBio.00693-16
Antagonism of the Sodium-Potassium ATPase Impairs Chikungunya Virus Infection
Abstract
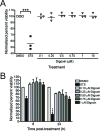
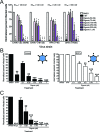
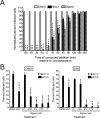
Chikungunya virus (CHIKV) is a reemerging alphavirus that has caused epidemics of fever, arthralgia, and rash worldwide. There are currently no licensed vaccines or antiviral therapies available for the prevention or treatment of CHIKV disease. We conducted a high-throughput, chemical compound screen that identified digoxin, a cardiac glycoside that blocks the sodium-potassium ATPase, as a potent inhibitor of CHIKV infection. Treatment of human cells with digoxin or a related cardiac glycoside, ouabain, resulted in a dose-dependent decrease in infection by CHIKV. Inhibition by digoxin was cell type-specific, as digoxin treatment of either murine or mosquito cells did not diminish CHIKV infection. Digoxin displayed antiviral activity against other alphaviruses, including Ross River virus and Sindbis virus, as well as mammalian reovirus and vesicular stomatitis virus. The digoxin-mediated block to CHIKV and reovirus infection occurred at one or more postentry steps, as digoxin inhibition was not bypassed by fusion of CHIKV at the plasma membrane or infection with cell surface-penetrating reovirus entry intermediates. Selection of digoxin-resistant CHIKV variants identified multiple mutations in the nonstructural proteins required for replication complex formation and synthesis of viral RNA. These data suggest a role for the sodium-potassium ATPase in promoting postentry steps of CHIKV replication and provide rationale for modulation of this pathway as a broad-spectrum antiviral strategy.
Importance: Mitigation of disease induced by globally spreading, mosquito-borne arthritogenic alphaviruses requires the development of new antiviral strategies. High-throughput screening of clinically tested compounds provides a rapid means to identify undiscovered, antiviral functions for well-characterized therapeutics and illuminate host pathways required for viral infection. Our study describes the potent inhibition of Chikungunya virus and related alphaviruses by the cardiac glycoside digoxin and demonstrates a function for the sodium-potassium ATPase in Chikungunya virus infection.
Copyright © 2016 Ashbrook et al.
Figures





References
-
- Simon F, Parola P, Grandadam M, Fourcade S, Oliver M, Brouqui P, Hance P, Kraemer P, Ali Mohamed A, de Lamballerie X, Charrel R, Tolou H. 2007. Chikungunya infection: an emerging rheumatism among travelers returned from Indian Ocean islands. Report of 47 cases. Medicine (Baltimore) 86:123–137. doi: 10.1097/MD/0b013e31806010a5. - DOI - PubMed
-
- Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, Cordioli P, Fortuna C, Boros S, Magurano F, Silvi G, Angelini P, Dottori M, Ciufolini MG, Majori GC, Cassone A, CHIKV Study Group . 2007. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet 370:1840–1846. doi: 10.1016/S0140-6736(07)61779-6. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
