Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma
- PMID: 27222480
- PMCID: PMC4957162
- DOI: 10.1182/blood-2015-12-687749
Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma
Abstract
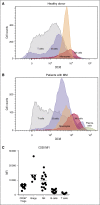
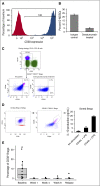
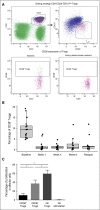
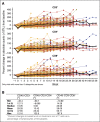
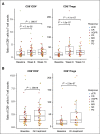
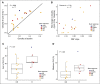
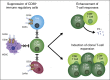
Daratumumab targets CD38-expressing myeloma cells through a variety of immune-mediated mechanisms (complement-dependent cytotoxicity, antibody-dependent cell-mediated cytotoxicity, and antibody-dependent cellular phagocytosis) and direct apoptosis with crosslinking. These mechanisms may also target nonplasma cells that express CD38, which prompted evaluation of daratumumab's effects on CD38-positive immune subpopulations. Peripheral blood (PB) and bone marrow (BM) from patients with relapsed/refractory myeloma from 2 daratumumab monotherapy studies were analyzed before and during therapy and at relapse. Regulatory B cells and myeloid-derived suppressor cells, previously shown to express CD38, were evaluated for immunosuppressive activity and daratumumab sensitivity in the myeloma setting. A novel subpopulation of regulatory T cells (Tregs) expressing CD38 was identified. These Tregs were more immunosuppressive in vitro than CD38-negative Tregs and were reduced in daratumumab-treated patients. In parallel, daratumumab induced robust increases in helper and cytotoxic T-cell absolute counts. In PB and BM, daratumumab induced significant increases in CD8(+):CD4(+) and CD8(+):Treg ratios, and increased memory T cells while decreasing naïve T cells. The majority of patients demonstrated these broad T-cell changes, although patients with a partial response or better showed greater maximum effector and helper T-cell increases, elevated antiviral and alloreactive functional responses, and significantly greater increases in T-cell clonality as measured by T-cell receptor (TCR) sequencing. Increased TCR clonality positively correlated with increased CD8(+) PB T-cell counts. Depletion of CD38(+) immunosuppressive cells, which is associated with an increase in T-helper cells, cytotoxic T cells, T-cell functional response, and TCR clonality, represents possible additional mechanisms of action for daratumumab and deserves further exploration.
© 2016 by The American Society of Hematology.
Figures
Comment in
-
A new era of immune therapy in multiple myeloma.Blood. 2016 Jul 21;128(3):318-9. doi: 10.1182/blood-2016-06-719856. Blood. 2016. PMID: 27445408 No abstract available.
References
-
- Harousseau JL. Ten years of improvement in the management of multiple myeloma: 2000-2010. Clin Lymphoma Myeloma Leuk. 2010;10(6):424–442. - PubMed
-
- Kastritis E, Zervas K, Symeonidis A, et al. Improved survival of patients with multiple myeloma after the introduction of novel agents and the applicability of the International Staging System (ISS): an analysis of the Greek Myeloma Study Group (GMSG). Leukemia. 2009;23(6):1152–1157. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

