Design strategies to address the effect of hydrophobic epitope on stability and in vitro assembly of modular virus-like particle
- PMID: 27222486
- PMCID: PMC4972206
- DOI: 10.1002/pro.2953
Design strategies to address the effect of hydrophobic epitope on stability and in vitro assembly of modular virus-like particle
Abstract
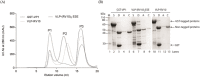
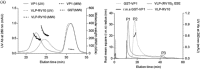
Virus-like particles (VLPs) and capsomere subunits have shown promising potential as safe and effective vaccine candidates. They can serve as platforms for the display of foreign epitopes on their surfaces in a modular architecture. Depending on the physicochemical properties of the antigenic modules, modularization may affect the expression, solubility and stability of capsomeres, and VLP assembly. In this study, three module designs of a rotavirus hydrophobic peptide (RV10) were synthesized using synthetic biology. Among the three synthetic modules, modularization of the murine polyomavirus VP1 with a single copy of RV10 flanked by long linkers and charged residues resulted in the expression of stable modular capsomeres. Further employing the approach of module titration of RV10 modules on each capsomere via Escherichia coli co-expression of unmodified VP1 and modular VP1-RV10 successfully translated purified modular capomeres into modular VLPs when assembled in vitro. Our results demonstrate that tailoring the physicochemical properties of modules to enhance modular capsomeres stability is achievable through synthetic biology designs. Combined with module titration strategy to avoid steric hindrance to intercapsomere interactions, this allows bioprocessing of bacterially produced in vitro assembled modular VLPs.
Keywords: Escherichia coli; co-expression; linkers; module titration; rotavirus; synthetic biology.
© 2016 The Protein Society.
Figures



Similar articles
-
Integrated molecular and bioprocess engineering for bacterially produced immunogenic modular virus-like particle vaccine displaying 18 kDa rotavirus antigen.Biotechnol Bioeng. 2017 Feb;114(2):397-406. doi: 10.1002/bit.26068. Epub 2016 Aug 17. Biotechnol Bioeng. 2017. PMID: 27497268
-
Synthetic biology design to display an 18 kDa rotavirus large antigen on a modular virus-like particle.Vaccine. 2015 Nov 4;33(44):5937-44. doi: 10.1016/j.vaccine.2015.09.017. Epub 2015 Sep 19. Vaccine. 2015. PMID: 26387437
-
A microbial platform for rapid and low-cost virus-like particle and capsomere vaccines.Vaccine. 2011 Sep 22;29(41):7154-62. doi: 10.1016/j.vaccine.2011.05.075. Epub 2011 Jun 7. Vaccine. 2011. PMID: 21651936
-
Production and biomedical applications of virus-like particles derived from polyomaviruses.J Control Release. 2013 Nov 28;172(1):305-321. doi: 10.1016/j.jconrel.2013.08.026. Epub 2013 Aug 31. J Control Release. 2013. PMID: 23999392 Review.
-
Lessons learned from successful human vaccines: Delineating key epitopes by dissecting the capsid proteins.Hum Vaccin Immunother. 2015;11(5):1277-92. doi: 10.1080/21645515.2015.1016675. Hum Vaccin Immunother. 2015. PMID: 25751641 Free PMC article. Review.
Cited by
-
An in-silico study of the mutation-associated effects on the spike protein of SARS-CoV-2, Omicron variant.PLoS One. 2022 Apr 21;17(4):e0266844. doi: 10.1371/journal.pone.0266844. eCollection 2022. PLoS One. 2022. PMID: 35446879 Free PMC article.
-
Proteomic Approach for Comparative Analysis of the Spike Protein of SARS-CoV-2 Omicron (B.1.1.529) Variant and Other Pango Lineages.Proteomes. 2022 Oct 14;10(4):34. doi: 10.3390/proteomes10040034. Proteomes. 2022. PMID: 36278694 Free PMC article.
-
MS2 Virus-like Particles as a Versatile Peptide Presentation Platform: Insights into the Deterministic Abilities for Accommodating Heterologous Peptide Lengths.ACS Synth Biol. 2023 Dec 15;12(12):3704-3715. doi: 10.1021/acssynbio.3c00503. Epub 2023 Nov 9. ACS Synth Biol. 2023. PMID: 37946498 Free PMC article.
-
Retrospective study, full-length genome characterization and evaluation of viral infectivity and pathogenicity of chimeric porcine deltacoronavirus detected in Vietnam.Transbound Emerg Dis. 2020 Jan;67(1):183-198. doi: 10.1111/tbed.13339. Epub 2019 Sep 13. Transbound Emerg Dis. 2020. PMID: 31469947 Free PMC article.
References
-
- Lua LHL, Connors NK, Sainsbury F, Chuan YP, Wibowo N, Middelberg APJ (2014) Bioengineering virus like‐particles as vaccines. Biotechnol Bioeng 111:425–440. - PubMed
-
- Adkins J, AJ W (1998) Recombinant hepatitis B vaccine: a review of its immunogenicity and protective efficacy against hepatitis B. Biodrugs 10:137–158. - PubMed
-
- Keating G, Noble S (2003) Recombinant hepatitis B vaccine (Engerix‐B): a review of its immunogenicity and protective efficacy against hepatitis B. Drugs 63:1021–1051. - PubMed
-
- Siddiqui M, Perry C (2006) Human papillomavirus quadrivalent (types 6, 11, 16, 18) recombinant vaccine (Gardasil). Drugs 66:1263–1271. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

