Mechanisms of Diabetes Improvement Following Bariatric/Metabolic Surgery
- PMID: 27222547
- PMCID: PMC5864134
- DOI: 10.2337/dc16-0145
Mechanisms of Diabetes Improvement Following Bariatric/Metabolic Surgery
Abstract
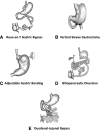
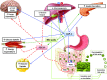
More than 20 years ago, Pories et al. published a seminal article, "Who Would Have Thought It? An Operation Proves to Be the Most Effective Therapy for Adult-Onset Diabetes Mellitus." This was based on their observation that bariatric surgery rapidly normalized blood glucose levels in obese people with type 2 diabetes mellitus (T2DM), and 10 years later, almost 90% remained diabetes free. Pories et al. suggested that caloric restriction played a key role and that the relative contributions of proximal intestinal nutrient exclusion, rapid distal gut nutrient delivery, and the role of gut hormones required further investigation. These findings of T2DM improvement/remission after bariatric surgery have been widely replicated, together with the observation that bariatric surgery prevents or delays incident T2DM. Over the ensuing two decades, important glucoregulatory roles of the gastrointestinal (GI) tract have been firmly established. However, the physiological and molecular mechanisms underlying the beneficial glycemic effects of bariatric surgery remain incompletely understood. In addition to the mechanisms proposed by Pories et al., changes in bile acid metabolism, GI tract nutrient sensing and glucose utilization, incretins, possible anti-incretin(s), and the intestinal microbiome are implicated. These changes, acting through peripheral and/or central pathways, lead to reduced hepatic glucose production, increased tissue glucose uptake, improved insulin sensitivity, and enhanced β-cell function. A constellation of factors, rather than a single overarching mechanism, likely mediate postoperative glycemic improvement, with the contributing factors varying according to the surgical procedure. Thus, different bariatric/metabolic procedures provide us with experimental tools to probe GI tract physiology. Embracing this approach through the application of detailed phenotyping, genomics, metabolomics, and gut microbiome studies will enhance our understanding of metabolic regulation and help identify novel therapeutic targets.
© 2016 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures

References
-
- Rutter GA. Dorothy Hodgkin Lecture 2014. Understanding genes identified by genome-wide association studies for type 2 diabetes. Diabet Med 2014;31:1480–1487 - PubMed
-
- Barnes CG. Hypoglycaemia following partial gastrectomy; report of three cases. Lancet 1947;2:536–539 - PubMed
-
- Evensen OK. Alimentary hypoglycemia after stomach operations and influence of gastric emptying on glucose tolerance curve. Acta Med Scand 1942;110:143–153
-
- Herbst CA, Hughes TA, Gwynne JT, Buckwalter JA. Gastric bariatric operation in insulin-treated adults. Surgery 1984;95:209–214 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

