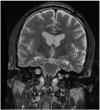
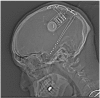
Mitigating bit flips or single event upsets in epilepsy neurostimulators
- PMID: 27222798
- PMCID: PMC4872716
- DOI: 10.1016/j.ebcr.2016.04.002
Mitigating bit flips or single event upsets in epilepsy neurostimulators
Abstract
Objectives: The objective of this study was to review software errors known as single event upsets (SEUs) or bit flips due to cosmic rays in epilepsy neurostimulators.
Materials and methods: A case report of a single event upset or bit flip is discussed; device manufacturers and publicly available data were queried for both incidence and types of error as well as strategies of software error mitigation.
Results: Neurostimulators, like other implanted devices such as pacemakers, are prone to single event upsets. Strategies for SEU mitigation are reviewed.
Conclusions: Cosmic radiation can threaten RAM and settings of neurostimulators; neuromodulation teams and device designers need to take this threat into account when designing multifunctional neuromodulation systems.
Keywords: Cosmic; Epilepsy; Neurostimulation; Neutron; Safety; Software.
Figures
References
-
- Ferrick A.M., Bernstein N., Aizer A., Chinitz L. Cosmic radiation induced software electrical resets in ICDs during air travel. Heart Rhythm. 2008;5(8):1201–1203. [Available from: http://www.sciencedirect.com/science/article/pii/S1547527108004815] - PubMed
-
- Baumann R.C. Soft errors in advanced semiconductor devices—part I: the three radiation sources. IEEE Trans Device Mater Reliab. 2001;1(1):17–22. [Available from: https://www.researchgate.net/publication/3429937_Soft_errors_in_advanced...]
-
- Bradley P.D., Normand E. Single event upsets in implantable cardioverter defibrillators. IEEE Trans Nucl Sci. 1998;45(6):2929–2940. [Available from: http://www.uow.edu.au/~pbradley/publications/SEUinICD.pdf]
-
- Trigano A., Hubert G., Marfaing J., Castellani K. Experimental study of neutron-induced soft errors in modern cardiac pacemakers. J Interv Card Electrophysiol. 2011;33(1):19–25. [Available from: http://link.springer.com/article/10.1007%2Fs10840-011-9609-6] - PubMed
-
- Manufacturer and User Facility Device Experience (MAUDE) Database [Internet]. Maryland: FDA U.S. Food and Drug Administration; [date unknown] [updated 2016 February 29; cited 2–16 January 9] Available from: www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/TextResults.cfm.
LinkOut - more resources
Full Text Sources
Other Literature Sources