Activated dendritic cells delivered in tissue compatible biomatrices induce in-situ anti-tumor CTL responses leading to tumor regression
- PMID: 27223090
- PMCID: PMC5129979
- DOI: 10.18632/oncotarget.9529
Activated dendritic cells delivered in tissue compatible biomatrices induce in-situ anti-tumor CTL responses leading to tumor regression
Abstract
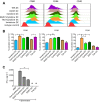
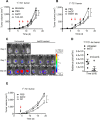
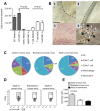
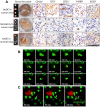
Dendritic cell (DC) based anti-cancer immunotherapy is well tolerated in patients with advanced cancers. However, the clinical responses seen after adoptive DC therapy have been suboptimal. Several factors including scarce DC numbers in tumors and immunosuppressive tumor microenvironments contribute to the inefficacy of DCs as cellular vaccines. Hence DC based vaccines can benefit from novel methods of cell delivery that would prevent the direct exposure of immune cells to suppressive tumor microenvironments. Here we evaluated the ability of DCs harbored in biocompatible scaffolds (referred to as biomatrix entrapped DCs; beDCs) in activating specific anti-tumor immune responses against primary and post-surgery secondary tumors. Using a preclinical cervical cancer and a melanoma model in mice, we show that single treatment of primary and post-surgery secondary tumors using beDCs resulted in significant tumor growth retardation while multiple inoculations were required to achieve a significant anti-tumor effect when DCs were given in free form. Additionally, we found that, compared to the tumor specific E6/E7 peptide vaccine, total tumor lysate induced higher expression of CD80 and CD40 on DCs that induced increased levels of IFNγ production upon interaction with host lymphocytes. Remarkably, a strong immunocyte infiltration into the host-implanted DC-scaffold was observed. Importantly, the host-implanted beDCs induced the anti-tumor immune responses in the absence of any stromal cell support, and the biomatrix structure was eventually absorbed into the surrounding host tissue. Collectively, these data indicate that the scaffold-based DC delivery may provide an efficient and safe way of delivering cell-based vaccines for treatment of primary and post-surgery secondary tumors.
Keywords: biomatrices; dendritic cells; immunotherapy; tumor.
Conflict of interest statement
None to declare.
Figures

Similar articles
-
Tumor-associated antigen/IL-21-transduced dendritic cell vaccines enhance immunity and inhibit immunosuppressive cells in metastatic melanoma.Gene Ther. 2014 May;21(5):457-67. doi: 10.1038/gt.2014.12. Epub 2014 Feb 27. Gene Ther. 2014. PMID: 24572790
-
The boosting effect of co-transduction with cytokine genes on cancer vaccine therapy using genetically modified dendritic cells expressing tumor-associated antigen.Int J Oncol. 2006 Apr;28(4):947-53. Int J Oncol. 2006. PMID: 16525645
-
Dendritic cell-tumor cell hybrids enhance the induction of cytotoxic T lymphocytes against murine colon cancer: a comparative analysis of antigen loading methods for the vaccination of immunotherapeutic dendritic cells.Oncol Rep. 2006 Dec;16(6):1317-24. Oncol Rep. 2006. PMID: 17089056
-
Dendritic Cells and Cancer Immunity.Trends Immunol. 2016 Dec;37(12):855-865. doi: 10.1016/j.it.2016.09.006. Epub 2016 Oct 25. Trends Immunol. 2016. PMID: 27793569 Free PMC article. Review.
-
Dendritic cells and cancer immunotherapy.Curr Opin Immunol. 2014 Apr;27:26-32. doi: 10.1016/j.coi.2014.01.005. Epub 2014 Feb 8. Curr Opin Immunol. 2014. PMID: 24513968 Review.
Cited by
-
Synthetic immune niches for cancer immunotherapy.Nat Rev Immunol. 2018 Mar;18(3):212-219. doi: 10.1038/nri.2017.89. Epub 2017 Aug 30. Nat Rev Immunol. 2018. PMID: 28853444 Review.
-
Cell and tissue engineering in lymph nodes for cancer immunotherapy.Adv Drug Deliv Rev. 2020;161-162:42-62. doi: 10.1016/j.addr.2020.07.023. Epub 2020 Aug 1. Adv Drug Deliv Rev. 2020. PMID: 32750376 Free PMC article.
-
Biomaterials for enhanced immunotherapy.APL Bioeng. 2022 Dec 16;6(4):041502. doi: 10.1063/5.0125692. eCollection 2022 Dec. APL Bioeng. 2022. PMID: 36561511 Free PMC article. Review.
-
Advances in immunotherapy delivery from implantable and injectable biomaterials.Acta Biomater. 2019 Apr 1;88:15-31. doi: 10.1016/j.actbio.2019.02.016. Epub 2019 Feb 13. Acta Biomater. 2019. PMID: 30771535 Free PMC article. Review.
-
Antitumour dendritic cell vaccination in a priming and boosting approach.Nat Rev Drug Discov. 2020 Sep;19(9):635-652. doi: 10.1038/s41573-020-0074-8. Epub 2020 Aug 6. Nat Rev Drug Discov. 2020. PMID: 32764681 Review.
References
-
- DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. - PubMed
-
- de Rosa F, Ridolfi L, Ridolfi R, Gentili G, Valmorri L, Nanni O, Petrini M, Fiammenghi L, Granato AM, Ancarani V, Pancisi E, Soldati V, Cassan S, et al. Vaccination with autologous dendritic cells loaded with autologous tumor lysate or homogenate combined with immunomodulating radiotherapy and/or preleukapheresis IFN-alpha in patients with metastatic melanoma: a randomised “proof-of-principle” phase II study. J Transl Med. 2014;12:209. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

