Fibrous Hydrogels for Cell Encapsulation: A Modular and Supramolecular Approach
- PMID: 27223105
- PMCID: PMC4880210
- DOI: 10.1371/journal.pone.0155625
Fibrous Hydrogels for Cell Encapsulation: A Modular and Supramolecular Approach
Erratum in
-
Correction: Fibrous Hydrogels for Cell Encapsulation: A Modular and Supramolecular Approach.PLoS One. 2016 Jul 18;11(7):e0159893. doi: 10.1371/journal.pone.0159893. eCollection 2016. PLoS One. 2016. PMID: 27427760 Free PMC article.
Abstract
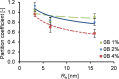
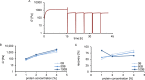
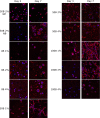
Artificial 3-dimensional (3D) cell culture systems, which mimic the extracellular matrix (ECM), hold great potential as models to study cellular processes under controlled conditions. The natural ECM is a 3D structure composed of a fibrous hydrogel that provides both mechanical and biochemical cues to instruct cell behavior. Here we present an ECM-mimicking genetically engineered protein-based hydrogel as a 3D cell culture system that combines several key features: (1) Mild and straightforward encapsulation meters (1) ease of ut I am not so sure.encapsulation of the cells, without the need of an external crosslinker. (2) Supramolecular assembly resulting in a fibrous architecture that recapitulates some of the unique mechanical characteristics of the ECM, i.e. strain-stiffening and self-healing behavior. (3) A modular approach allowing controlled incorporation of the biochemical cue density (integrin binding RGD domains). We tested the gels by encapsulating MG-63 osteoblastic cells and found that encapsulated cells not only respond to higher RGD density, but also to overall gel concentration. Cells in 1% and 2% (weight fraction) protein gels showed spreading and proliferation, provided a relative RGD density of at least 50%. In contrast, in 4% gels very little spreading and proliferation occurred, even for a relative RGD density of 100%. The independent control over both mechanical and biochemical cues obtained in this modular approach renders our hydrogels suitable to study cellular responses under highly defined conditions.
Conflict of interest statement
Figures






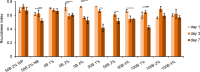
Similar articles
-
Independent control of matrix adhesiveness and stiffness within a 3D self-assembling peptide hydrogel.Acta Biomater. 2018 Apr 1;70:110-119. doi: 10.1016/j.actbio.2018.01.031. Epub 2018 Feb 2. Acta Biomater. 2018. PMID: 29410241
-
Electrostatic Assembly of Multiarm PEG-Based Hydrogels as Extracellular Matrix Mimics: Cell Response in the Presence and Absence of RGD Cell Adhesive Ligands.ACS Biomater Sci Eng. 2023 Mar 13;9(3):1362-1376. doi: 10.1021/acsbiomaterials.2c01252. Epub 2023 Feb 24. ACS Biomater Sci Eng. 2023. PMID: 36826383
-
Kidney decellularized extracellular matrix hydrogels: Rheological characterization and human glomerular endothelial cell response to encapsulation.J Biomed Mater Res A. 2018 Sep;106(9):2448-2462. doi: 10.1002/jbm.a.36439. J Biomed Mater Res A. 2018. PMID: 29664217 Free PMC article.
-
Viscoelastic hydrogels for 3D cell culture.Biomater Sci. 2017 Jul 25;5(8):1480-1490. doi: 10.1039/c7bm00261k. Biomater Sci. 2017. PMID: 28584885 Review.
-
Synthesis and high-throughput processing of polymeric hydrogels for 3D cell culture.Bioconjug Chem. 2014 Sep 17;25(9):1581-601. doi: 10.1021/bc500310v. Epub 2014 Sep 4. Bioconjug Chem. 2014. PMID: 25153031 Review.
Cited by
-
Inducible Fibril Formation of Silk-Elastin Diblocks.ACS Omega. 2019 May 31;4(5):9135-9143. doi: 10.1021/acsomega.9b01025. Epub 2019 May 23. ACS Omega. 2019. PMID: 31172045 Free PMC article.
-
Self-Healing Injectable Hydrogels for Tissue Regeneration.Chem Rev. 2023 Jan 25;123(2):834-873. doi: 10.1021/acs.chemrev.2c00179. Epub 2022 Aug 5. Chem Rev. 2023. PMID: 35930422 Free PMC article. Review.
-
Production of protein-based polymers in Pichia pastoris.Biotechnol Adv. 2019 Sep-Oct;37(5):642-666. doi: 10.1016/j.biotechadv.2019.03.012. Epub 2019 Mar 19. Biotechnol Adv. 2019. PMID: 30902728 Free PMC article. Review.
-
Correction: Fibrous Hydrogels for Cell Encapsulation: A Modular and Supramolecular Approach.PLoS One. 2016 Jul 18;11(7):e0159893. doi: 10.1371/journal.pone.0159893. eCollection 2016. PLoS One. 2016. PMID: 27427760 Free PMC article.
-
In-Silico Modelling of Transdermal Delivery of Macromolecule Drugs Assisted by a Skin Stretching Hypobaric Device.Pharm Res. 2023 Jan;40(1):295-305. doi: 10.1007/s11095-022-03423-7. Epub 2022 Nov 8. Pharm Res. 2023. PMID: 36348132 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

