Platelet factor 4 is produced by subsets of myeloid cells in premetastatic lung and inhibits tumor metastasis
- PMID: 27223426
- PMCID: PMC5438604
- DOI: 10.18632/oncotarget.9486
Platelet factor 4 is produced by subsets of myeloid cells in premetastatic lung and inhibits tumor metastasis
Abstract
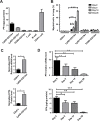
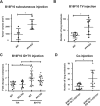
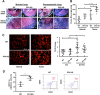
Bone marrow-derived myeloid cells can form a premetastatic niche and provide a tumor-promoting microenvironment. However, subsets of myeloid cells have also been reported to have anti-tumor properties. It is not clear whether there is a transition between anti- and pro- tumor function of these myeloid cells, and if so, what are the underlying molecular mechanisms. Here we report platelet factor 4 (PF4), or CXCL4, but not the other family members CXCL9, 10, and 11, was produced at higher levels in the normal lung and early stage premetastatic lungs but decreased in later stage lungs. PF4 was mostly produced by Ly6G+CD11b+ myeloid cell subset. Although the number of Ly6G+CD11b+ cells was increased in the premetastatic lungs, the expression level of PF4 in these cells was decreased during the metastatic progression. Deletion of PF4 (PF4 knockout or KO mice) led an increased metastasis suggesting an inhibitory function of PF4. There were two underlying mechanisms: decreased blood vessel integrity in the premetastatic lungs and increased production of hematopoietic stem/progenitor cells (HSCs) and myeloid derived suppressor cells (MDSCs) in tumor-bearing PF4 KO mice. In cancer patients, PF4 expression levels were negatively correlated with tumor stage and positively correlated with patient survival. Our studies suggest that PF4 is a critical anti-tumor factor in the premetastatic site. Our finding of PF4 function in the tumor host provides new insight to the mechanistic understanding of tumor metastasis.
Keywords: PF4; metastasis; myeloid cells; premetastatic lung.
Conflict of interest statement
The authors declare no competing financial interest.
The research work was supported by NCI intramural funding to Drs. Li Yang, and the Collaborative Research of National Institutes of Health-Natural Science Foundation of China (Nos.81361120399) to Xinhua Liang.
Figures



References
-
- Mantovani A. Cancer: Inflaming metastasis. Nature. 2009;457:36–37. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

