Pharmacometabolomics study identifies circulating spermidine and tryptophan as potential biomarkers associated with the complete pathological response to trastuzumab-paclitaxel neoadjuvant therapy in HER-2 positive breast cancer
- PMID: 27223427
- PMCID: PMC5129972
- DOI: 10.18632/oncotarget.9489
Pharmacometabolomics study identifies circulating spermidine and tryptophan as potential biomarkers associated with the complete pathological response to trastuzumab-paclitaxel neoadjuvant therapy in HER-2 positive breast cancer
Abstract
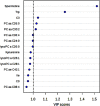
Defining biomarkers that predict therapeutic effects and adverse events is a crucial mandate to guide patient selection for personalized cancer treatments. In the present study, we applied a pharmacometabolomics approach to identify biomarkers potentially associated with pathological complete response to trastuzumab-paclitaxel neoadjuvant therapy in HER-2 positive breast cancer patients. Based on histological response the 34 patients enrolled in the study were subdivided into two groups: good responders (n = 15) and poor responders (n = 19). The pre-treatment serum targeted metabolomics profile of all patients were analyzed by liquid chromatography tandem mass spectrometry and the differences in the metabolomics profile between the two groups was investigated by multivariate partial least squares discrimination analysis. The most relevant metabolites that differentiate the two groups of patients were spermidine and tryptophan. The Good responders showed higher levels of spermidine and lower amounts of tryptophan compared with the poor responders (p < 0.001, q < 0.05). The serum level of these two metabolites identified patients who achieved a pathological complete response with a sensitivity of 90% [0.79-1.00] and a specificity of 0.87% [0.67-1.00]. These preliminary results support the role played by the individual patients' metabolism in determining the response to cancer treatments and may be a useful tool to select patients that are more likely to benefit from the trastuzumab-paclitaxel treatment.
Keywords: breast; cancer; metabolomics; pharmacometabolomics; pharmacometabonomics.
Conflict of interest statement
There are no conflicts of interests to declare.
Figures






References
-
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. - PubMed
-
- Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, de AE, Procter M, Suter TM, Jackisch C, Cameron D, Weber HA, Heinzmann D, Dal LL, McFadden E, Dowsett M, et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet. 2013;382:1021–1028. - PubMed
-
- Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, Goldhirsch A, Untch M, Mariani G, Baselga J, Kaufmann M, Cameron D, Bell R, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. - PubMed
-
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. - PubMed
-
- Gianni L, Eiermann W, Semiglazov V, Lluch A, Tjulandin S, Zambetti M, Moliterni A, Vazquez F, Byakhov MJ, Lichinitser M, Climent MA, Ciruelos E, Ojeda B, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014;15:640–647. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

