Mechanisms Involved in the Development of the Chronic Gastrointestinal Syndrome in Nonhuman Primates after Total-Body Irradiation with Bone Marrow Shielding
- PMID: 27223826
- PMCID: PMC4966619
- DOI: 10.1667/RR14024.1
Mechanisms Involved in the Development of the Chronic Gastrointestinal Syndrome in Nonhuman Primates after Total-Body Irradiation with Bone Marrow Shielding
Abstract
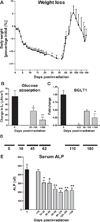
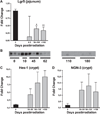
In this study, nonhuman primates (NHPs) exposed to lethal doses of total body irradiation (TBI) within the gastrointestinal (GI) acute radiation syndrome range, sparing ∼5% of bone marrow (TBI-BM5), were used to evaluate the mechanisms involved in development of the chronic GI syndrome. TBI increased mucosal permeability in the jejunum (12-14 Gy) and proximal colon (13-14 Gy). TBI-BM5 also impaired mucosal barrier function at doses ranging from 10-12.5 Gy in both small intestine and colon. Timed necropsies of NHPs at 6-180 days after 10 Gy TBI-BM5 showed that changes in small intestine preceded those in the colon. Chronic GI syndrome in NHPs is characterized by continued weight loss and intermittent GI syndrome symptoms. There was a long-lasting decrease in jejunal glucose absorption coincident with reduced expression of the sodium-linked glucose transporter. The small intestine and colon showed a modest upregulation of several different pro-inflammatory mediators such as NOS-2. The persistent inflammation in the post-TBI-BM5 period was associated with a long-lasting impairment of mucosal restitution and a reduced expression of intestinal and serum levels of alkaline phosphatase (ALP). Mucosal healing in the postirradiation period is dependent on sparing of stem cell crypts and maturation of crypt cells into appropriate phenotypes. At 30 days after 10 Gy TBI-BM5, there was a significant downregulation in the gene and protein expression of the stem cell marker Lgr5 but no change in the gene expression of enterocyte or enteroendocrine lineage markers. These data indicate that even a threshold dose of 10 Gy TBI-BM5 induces a persistent impairment of both mucosal barrier function and restitution in the GI tract and that ALP may serve as a biomarker for these events. These findings have important therapeutic implications for the design of medical countermeasures.
Figures








Similar articles
-
Evaluation of Plasma Biomarker Utility for the Gastrointestinal Acute Radiation Syndrome in Non-human Primates after Partial Body Irradiation with Minimal Bone Marrow Sparing through Correlation with Tissue and Histological Analyses.Health Phys. 2020 Nov;119(5):594-603. doi: 10.1097/HP.0000000000001348. Health Phys. 2020. PMID: 32947487 Free PMC article.
-
Morphological and functional impairment in the gut in a partial body irradiation minipig model of GI-ARS.Int J Radiat Biol. 2020 Jan;96(1):112-128. doi: 10.1080/09553002.2018.1552377. Epub 2019 Jan 7. Int J Radiat Biol. 2020. PMID: 30475652
-
Development of A Novel Murine Model of Combined Radiation and Peripheral Tissue Trauma Injuries.Radiat Res. 2017 Feb;187(2):241-250. doi: 10.1667/RR14557.1. Epub 2017 Jan 24. Radiat Res. 2017. PMID: 28118112 Free PMC article.
-
Acute Radiation-induced Lung Injury in the Non-human Primate: A Review and Comparison of Mortality and Co-morbidities Using Models of Partial-body Irradiation with Marginal Bone Marrow Sparing and Whole Thorax Lung Irradiation.Health Phys. 2020 Nov;119(5):559-587. doi: 10.1097/HP.0000000000001346. Health Phys. 2020. PMID: 33009295 Free PMC article. Review.
-
[The gastrointestinal post-irradiation syndrome].Sb Ved Pr Lek Fak Karlovy Univerzity Hradci Kralove Suppl. 1991;34(4):477-503. Sb Ved Pr Lek Fak Karlovy Univerzity Hradci Kralove Suppl. 1991. PMID: 1815346 Review. Czech.
Cited by
-
Differential Recovery of Small Intestinal Segments after Partial-Body Irradiation in Non-Human Primates.Radiat Res. 2021 Aug 1;196(2):204-212. doi: 10.1667/RADE-20-00272.1. Radiat Res. 2021. PMID: 34043805 Free PMC article.
-
A High Throughput Approach to Reconstruct Partial-Body and Neutron Radiation Exposures on an Individual Basis.Sci Rep. 2020 Feb 19;10(1):2899. doi: 10.1038/s41598-020-59695-9. Sci Rep. 2020. PMID: 32076014 Free PMC article.
-
Characterizing the Natural History of Acute Radiation Syndrome of the Gastrointestinal Tract: Combining High Mass and Spatial Resolution Using MALDI-FTICR-MSI.Health Phys. 2019 Apr;116(4):454-472. doi: 10.1097/HP.0000000000000948. Health Phys. 2019. PMID: 30681424 Free PMC article.
-
Gas Chromatography/Mass Spectrometry Metabolomics of Urine and Serum from Nonhuman Primates Exposed to Ionizing Radiation: Impacts on the Tricarboxylic Acid Cycle and Protein Metabolism.J Proteome Res. 2017 May 5;16(5):2091-2100. doi: 10.1021/acs.jproteome.7b00064. Epub 2017 Apr 4. J Proteome Res. 2017. PMID: 28351153 Free PMC article.
-
Delayed Effects of Acute Radiation Exposure (Deare) in Juvenile and Old Rats: Mitigation by Lisinopril.Health Phys. 2019 Apr;116(4):529-545. doi: 10.1097/HP.0000000000000920. Health Phys. 2019. PMID: 30624354 Free PMC article.
References
-
- MacVittie TJ, Bennett A, Booth C, Garofalo M, Tudor G, Ward A, et al. The prolonged gastrointestinal syndrome in rhesus macaques: the relationship between gastrointestinal, hematopoietic, and delayed multi-organ sequelae following acute, potentially lethal, partial-body irradiation. Health Phys. 2012;103:427–453. - PMC - PubMed
-
- MacVittie TJ, Farese AM, Bennett A, Gelfond D, Shea-Donohue T, Tudor G, et al. The acute gastrointestinal subsyndrome of the acute radiation syndrome: a rhesus macaque model. Health Phys. 2012;103:411–426. - PubMed
-
- Lalles JP. Intestinal alkaline phosphatase: multiple biological roles in maintenance of intestinal homeostasis and modulation by diet. Nutr Rev. 2010;68:323–332. - PubMed
-
- Asmar RE, Panigrahi P, Bamford P, Berti I, Not T, Coppa GV, et al. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology. 2002;123:1607–1615. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

