Acyl-CoA:Diacylglycerol Acyltransferase 1 Expression Level in the Hematopoietic Compartment Impacts Inflammation in the Vascular Plaques of Atherosclerotic Mice
- PMID: 27223895
- PMCID: PMC4880185
- DOI: 10.1371/journal.pone.0156364
Acyl-CoA:Diacylglycerol Acyltransferase 1 Expression Level in the Hematopoietic Compartment Impacts Inflammation in the Vascular Plaques of Atherosclerotic Mice
Abstract
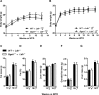
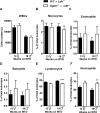
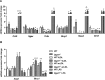
The final step of triacylglycerol synthesis is catalyzed by acyl-CoA:diacylglycerol acyltransferases (DGATs). We have previously shown that ApoE-/-Dgat1-/- mice are protected from developing atherosclerosis in association with reduced foam cell formation. However, the role of DGAT1, specifically in myeloid and other hematopoietic cell types, in determining this protective phenotype is unknown. To address this question, we reconstituted the bone marrow of irradiated Ldlr-/-mice with that from wild-type (WT→ Ldlr-/-) and Dgat1-/-(Dgat1-/-→ Ldlr-/-) donor mice. We noted that DGAT1 in the hematopoietic compartment exerts a sex-specific effect on systemic cholesterol homeostasis. However, both male and female Dgat1-/-→ Ldlr-/-mice had higher circulating neutrophil and lower lymphocyte counts than control mice, suggestive of a classical inflammatory phenotype. Moreover, specifically examining the aortae of these mice revealed that Dgat1-/-→ Ldlr-/-mice have atherosclerotic plaques with increased macrophage content. This increase was coupled to a reduced plaque collagen content, leading to a reduced collagen-to-macrophage ratio. Together, these findings point to a difference in the inflammatory contribution to plaque composition between Dgat1-/-→ Ldlr-/-and control mice. By contrast, DGAT1 deficiency did not affect the transcriptional responses of cultured macrophages to lipoprotein treatment in vitro, suggesting that the alterations seen in the plaques of Dgat1-/-→ Ldlr-/-mice in vivo do not reflect a cell intrinsic effect of DGAT1 in macrophages. We conclude that although DGAT1 in the hematopoietic compartment does not impact the overall lipid content of atherosclerotic plaques, it exerts reciprocal effects on inflammation and fibrosis, two processes that control plaque vulnerability.
Conflict of interest statement
Figures


Similar articles
-
Intestine-specific DGAT1 deficiency improves atherosclerosis in apolipoprotein E knockout mice by reducing systemic cholesterol burden.Atherosclerosis. 2020 Oct;310:26-36. doi: 10.1016/j.atherosclerosis.2020.07.030. Epub 2020 Aug 10. Atherosclerosis. 2020. PMID: 32882484 Free PMC article.
-
Lack of acyl-CoA:diacylglycerol acyltransferase 1 reduces intestinal cholesterol absorption and attenuates atherosclerosis in apolipoprotein E knockout mice.Biochim Biophys Acta. 2011 Dec;1811(12):1011-20. doi: 10.1016/j.bbalip.2011.08.010. Epub 2011 Sep 1. Biochim Biophys Acta. 2011. PMID: 21924378 Free PMC article.
-
Deficiency of Dab2 (Disabled Homolog 2) in Myeloid Cells Exacerbates Inflammation in Liver and Atherosclerotic Plaques in LDLR (Low-Density Lipoprotein Receptor)-Null Mice-Brief Report.Arterioscler Thromb Vasc Biol. 2018 May;38(5):1020-1029. doi: 10.1161/ATVBAHA.117.310467. Epub 2018 Mar 29. Arterioscler Thromb Vasc Biol. 2018. PMID: 29599136 Free PMC article.
-
Hematopoietic arginase 1 deficiency results in decreased leukocytosis and increased foam cell formation but does not affect atherosclerosis.Atherosclerosis. 2017 Jan;256:35-46. doi: 10.1016/j.atherosclerosis.2016.11.018. Epub 2016 Nov 16. Atherosclerosis. 2017. PMID: 27998825
-
Deficiency of circadian gene cryptochromes in bone marrow-derived cells protects against atherosclerosis in LDLR-/- mice.FASEB J. 2021 Feb;35(2):e21309. doi: 10.1096/fj.202001818RRR. FASEB J. 2021. PMID: 33423309
Cited by
-
The triglyceride synthesis enzymes DGAT1 and DGAT2 have distinct and overlapping functions in adipocytes.J Lipid Res. 2019 Jun;60(6):1112-1120. doi: 10.1194/jlr.M093112. Epub 2019 Apr 1. J Lipid Res. 2019. PMID: 30936184 Free PMC article.
-
Genetic deletion of MMP12 ameliorates cardiometabolic disease by improving insulin sensitivity, systemic inflammation, and atherosclerotic features in mice.Cardiovasc Diabetol. 2023 Nov 28;22(1):327. doi: 10.1186/s12933-023-02064-3. Cardiovasc Diabetol. 2023. PMID: 38017481 Free PMC article.
-
Lanifibranor Reduces Inflammation and Improves Dyslipidemia in Lysosomal Acid Lipase-Deficient Mice.Gastro Hep Adv. 2024 May 28;3(6):711-723. doi: 10.1016/j.gastha.2024.05.006. eCollection 2024. Gastro Hep Adv. 2024. PMID: 39280921 Free PMC article.
-
Intestine-specific DGAT1 deficiency improves atherosclerosis in apolipoprotein E knockout mice by reducing systemic cholesterol burden.Atherosclerosis. 2020 Oct;310:26-36. doi: 10.1016/j.atherosclerosis.2020.07.030. Epub 2020 Aug 10. Atherosclerosis. 2020. PMID: 32882484 Free PMC article.
References
-
- Cases S, Stone SJ, Zhou P, Yen E, Tow B, Lardizabal KD, et al. Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J Biol Chem. 2001;276(42):38870–6. - PubMed
-
- Stone SJ, Myers HM, Watkins SM, Brown BE, Feingold KR, Elias PM, et al. Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J Biol Chem. 2004;279(12):11767–76. - PubMed
-
- Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, Tow B, et al. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat Genet. 2000;25(1):87–90. - PubMed
-
- Chen HC, Ladha Z, Smith SJ, Farese RV. Analysis of energy expenditure at different ambient temperatures in mice lacking DGAT1. Am J Physiol-Endoc M. 2003;284(1):E213–E8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

