Increased translocation of antigens to endosomes and TLR4 mediated endosomal recruitment of TAP contribute to nicotine augmented cross-presentation
- PMID: 27224911
- PMCID: PMC5122403
- DOI: 10.18632/oncotarget.9498
Increased translocation of antigens to endosomes and TLR4 mediated endosomal recruitment of TAP contribute to nicotine augmented cross-presentation
Abstract
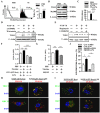
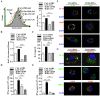
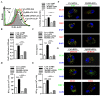
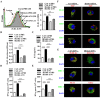
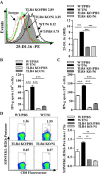
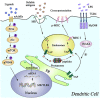
Cross-presentation by dendritic cells (DCs) requires surface molecules such as lectin, CD40, langerin, heat shock protein, mannose receptor, mediated endocytosis, the endosomal translocation of internalized antigen, and the relocation of transporter associated with antigen processing (TAP). Although the activation of α7 nicotinic acetylcholine receptor (α7 nAchR) up-regulate surface molecule expression, augment endocytosis, and enhance cross-presentation, the molecular mechanism of α7 nAchR activation-increased cross-presentation is still poorly understood. In this study, we investigated the role of mannose receptor in nicotine-increased cross-presentation and the mechanism that endotoxins orchestrating the recruitment of TAP toward endosomes. We demonstrated that nicotine increase the expressiones of mannose receptor and Toll-like receptor 4 (TLR4) via PI3K-Akt-mTOR-p70S6 pathway. Both endosomal translocation of mannose receptor-internalized antigens and TLR4 sig- naling are necessary for nicotine-augmented cross-presentation and cross-priming. Importantly, the recruitment of TAP toward endosomes via TLR4-MyD88-IRAK4 signaling contributes to nicotine-increased cross-presentation and cross-activation of T cells. Thus, these data suggest that increased recruitment of TAP to Ag-containing vesicles contributes to the superior cross-presentation efficacy of α7 nAchR activated DCs.
Keywords: cross-presentation; dendritic cells; mannose receptor; α7 nicotinic acetylcholine receptor; Toll-like receptor 4.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures


Similar articles
-
Mannose receptor polyubiquitination regulates endosomal recruitment of p97 and cytosolic antigen translocation for cross-presentation.Proc Natl Acad Sci U S A. 2011 Jun 14;108(24):9933-8. doi: 10.1073/pnas.1102397108. Epub 2011 May 31. Proc Natl Acad Sci U S A. 2011. PMID: 21628571 Free PMC article.
-
Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation.Nat Immunol. 2008 May;9(5):558-66. doi: 10.1038/ni.1601. Epub 2008 Mar 30. Nat Immunol. 2008. PMID: 18376402
-
Internalization and endosomal degradation of receptor-bound antigens regulate the efficiency of cross presentation by human dendritic cells.Blood. 2012 Sep 6;120(10):2011-20. doi: 10.1182/blood-2012-01-402370. Epub 2012 Jul 12. Blood. 2012. PMID: 22791285
-
Regulation of antigen transport into the cytosol for cross-presentation by ubiquitination of the mannose receptor.Mol Immunol. 2013 Sep;55(2):146-8. doi: 10.1016/j.molimm.2012.10.010. Epub 2012 Nov 3. Mol Immunol. 2013. PMID: 23127488 Review.
-
Spotlight on TAP and its vital role in antigen presentation and cross-presentation.Mol Immunol. 2022 Feb;142:105-119. doi: 10.1016/j.molimm.2021.12.013. Epub 2021 Dec 29. Mol Immunol. 2022. PMID: 34973498 Free PMC article. Review.
Cited by
-
PYR-41 and Thalidomide Impair Dendritic Cell Cross-Presentation by Inhibiting Myddosome Formation and Attenuating the Endosomal Recruitments of p97 and Sec61 via NF-κB Inactivation.J Immunol Res. 2018 Jul 5;2018:5070573. doi: 10.1155/2018/5070573. eCollection 2018. J Immunol Res. 2018. PMID: 30069488 Free PMC article.
-
Hypaphorine Attenuates Lipopolysaccharide-Induced Endothelial Inflammation via Regulation of TLR4 and PPAR-γ Dependent on PI3K/Akt/mTOR Signal Pathway.Int J Mol Sci. 2017 Apr 17;18(4):844. doi: 10.3390/ijms18040844. Int J Mol Sci. 2017. PMID: 28420166 Free PMC article.
-
Surface immunogenic protein from Streptococcus agalactiae and Fissurella latimarginata hemocyanin are TLR4 ligands and activate MyD88- and TRIF dependent signaling pathways.Front Immunol. 2023 Sep 18;14:1186188. doi: 10.3389/fimmu.2023.1186188. eCollection 2023. Front Immunol. 2023. PMID: 37790926 Free PMC article.
-
Autophagy Plays an Important Role in Anti-inflammatory Mechanisms Stimulated by Alpha7 Nicotinic Acetylcholine Receptor.Front Immunol. 2017 May 16;8:553. doi: 10.3389/fimmu.2017.00553. eCollection 2017. Front Immunol. 2017. PMID: 28559895 Free PMC article.
-
K48-Linked Ubiquitination Contributes to Nicotine-Augmented Bone Marrow-Derived Dendritic-Cell-Mediated Adaptive Immunity.Vaccines (Basel). 2021 Mar 19;9(3):278. doi: 10.3390/vaccines9030278. Vaccines (Basel). 2021. PMID: 33808531 Free PMC article.
References
-
- Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–8. - PubMed
-
- Villadangos JA, Heath WR, Carbone FR. Outside looking in: the inner workings of the cross-presentation pathway within dendritic cells. Trends Immunol. 2007;28:45–7. - PubMed
-
- Wessler IK, Kirkpatrick CJ. The non-neuronal cholinergic system: an emerging drug target in the airways. Pulm Pharmacol Ther. 2001;14:423–34. - PubMed
-
- Printz C. Gap narrows in African American smoking-related cancers, increases in breast and colorectal cancers. Cancer. 2011;117:2357. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

