Utility of patient-derived lymphoblastoid cell lines as an ex vivo capecitabine sensitivity prediction model for breast cancer patients
- PMID: 27224917
- PMCID: PMC5122395
- DOI: 10.18632/oncotarget.9521
Utility of patient-derived lymphoblastoid cell lines as an ex vivo capecitabine sensitivity prediction model for breast cancer patients
Abstract
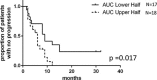
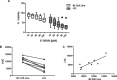
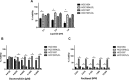
Capecitabine is commonly used in treating breast cancer; however, therapeutic response varies among patients and there is no clinically validated model to predict individual outcomes. Here, we investigated whether drug sensitivity quantified in ex vivo patients' blood-derived cell lines can predict response to capecitabine in vivo. Lymphoblastoid cell lines (LCLs) were established from a cohort of metastatic breast cancer patients (n = 53) who were prospectively monitored during treatment with single agent capecitabine at 2000 mg/m2/day. LCLs were treated with increasing concentrations of 5'-DFUR, a major capecitabine metabolite, to assess patients' ex vivo sensitivity to this drug. Subsequently, ex vivo phenotype was compared to observed patient disease response and drug induced-toxicities. We acquired an independent cohort of breast cancer cell lines and LCLs derived from the same donors from ATCC, compared their sensitivity to 5'-DFUR. As seen in the patient population, we observed large inter-individual variability in response to 5'-DFUR treatment in patient-derived LCLs. Patients whose LCLs were more sensitive to 5'-DFUR had a significantly longer median progression free survival (9-month vs 6-month, log rank p-value = 0.017). In addition, this significant positive correlation for 5'-DFUR sensitivity was replicated in an independent cohort of 8 breast cancer cell lines and LCLs derived from the same donor. Our data suggests that at least a portion of the individual sensitivity to capecitabine is shared between germline tissue and tumor tissue. It also supports the utility of patient-derived LCLs as a predictive model for capecitabine treatment efficacy in breast cancer patients.
Keywords: breast cancer; capecitabine; ex vivo model; lymphoblastoid cell lines; patient-derived model.
Conflict of interest statement
All authors have no conflicts of interest to disclose.
Figures
Similar articles
-
Pharmacokinetic and pharmacodynamic comparison of fluoropyrimidine derivatives, capecitabine and 5'-deoxy-5-fluorouridine (5'-DFUR).Cancer Chemother Pharmacol. 2005 Aug;56(2):205-11. doi: 10.1007/s00280-004-0934-7. Epub 2005 Apr 21. Cancer Chemother Pharmacol. 2005. PMID: 15844007 Clinical Trial.
-
Clinical efficacy of doxifluridine and correlation to in vitro sensitivity of anticancer drugs in patients with colorectal cancer.Anticancer Res. 2003 May-Jun;23(3B):2559-64. Anticancer Res. 2003. PMID: 12894541 Clinical Trial.
-
Efficacy of capecitabine monotherapy as the first-line treatment of metastatic HER2-negative breast cancer.Tumori. 2015 Jul-Aug;101(4):418-23. doi: 10.5301/tj.5000332. Tumori. 2015. PMID: 25953439 Clinical Trial.
-
Capecitabine: a review.Clin Ther. 2005 Jan;27(1):23-44. doi: 10.1016/j.clinthera.2005.01.005. Clin Ther. 2005. PMID: 15763604 Review.
-
A retrospective study evaluating a fixed low dose capecitabine monotherapy in women with HER-2 negative metastatic breast cancer.Breast Cancer Res Treat. 2014 Jul;146(1):7-14. doi: 10.1007/s10549-014-3003-x. Epub 2014 Jun 5. Breast Cancer Res Treat. 2014. PMID: 24899084 Review.
Cited by
-
Characterization of ascites-derived tumor cells from an endometrial cancer patient.Cancer Sci. 2017 Dec;108(12):2352-2357. doi: 10.1111/cas.13407. Epub 2017 Oct 25. Cancer Sci. 2017. PMID: 28945304 Free PMC article.
-
Exploring the Link between the Germline and Somatic Genome in Cancer.Cancer Discov. 2017 Apr;7(4):354-355. doi: 10.1158/2159-8290.CD-17-0192. Cancer Discov. 2017. PMID: 28373166 Free PMC article.
References
-
- Tabata T, Katoh M, Tokudome S, Hosakawa M, Chiba K, Nakajima M, Yokoi T. Bioactivation of capecitabine in human liver: involvement of the cytosolic enzyme on 5′-deoxy-5-fluorocytidine formation. Drug Metab Dispos. 2004;32:762–767. - PubMed
-
- Walko CM, Lindley C. Capecitabine: a review. Clin Ther. 2005;27:23–44. - PubMed
-
- Ershler WB. Capecitabine monotherapy: safe and effective treatment for metastatic breast cancer. Oncologist. 2006;11:325–335. - PubMed
-
- Reichardt P, Von Minckwitz G, Thuss-Patience PC, Jonat W, Kolbl H, Janicke F, Kieback DG, Kuhn W, Schindler AE, Mohrmann S, Kaufmann M, Luck HJ. Multicenter phase II study of oral capecitabine (Xeloda(“)) in patients with metastatic breast cancer relapsing after treatment with a taxane-containing therapy. Ann Oncol. 2003;14:1227–1233. - PubMed
-
- Blum JL, Jones SE, Buzdar AU, LoRusso PM, Kuter I, Vogel C, Osterwalder B, Burger HU, Brown CS, Griffin T. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol. 1999;17:485–493. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

