Synchronized mitochondrial and cytosolic translation programs
- PMID: 27225121
- PMCID: PMC4964289
- DOI: 10.1038/nature18015
Synchronized mitochondrial and cytosolic translation programs
Abstract
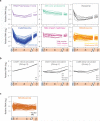
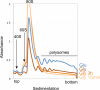
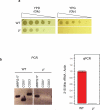
Oxidative phosphorylation (OXPHOS) is a vital process for energy generation, and is carried out by complexes within the mitochondria. OXPHOS complexes pose a unique challenge for cells because their subunits are encoded on both the nuclear and the mitochondrial genomes. Genomic approaches designed to study nuclear/cytosolic and bacterial gene expression have not been broadly applied to mitochondria, so the co-regulation of OXPHOS genes remains largely unexplored. Here we monitor mitochondrial and nuclear gene expression in Saccharomyces cerevisiae during mitochondrial biogenesis, when OXPHOS complexes are synthesized. We show that nuclear- and mitochondrial-encoded OXPHOS transcript levels do not increase concordantly. Instead, mitochondrial and cytosolic translation are rapidly, dynamically and synchronously regulated. Furthermore, cytosolic translation processes control mitochondrial translation unidirectionally. Thus, the nuclear genome coordinates mitochondrial and cytosolic translation to orchestrate the timely synthesis of OXPHOS complexes, representing an unappreciated regulatory layer shaping the mitochondrial proteome. Our whole-cell genomic profiling approach establishes a foundation for studies of global gene regulation in mitochondria.
Figures











Comment in
-
Cell biology: Choreography of protein synthesis.Nature. 2016 May 26;533(7604):472-3. doi: 10.1038/nature18436. Epub 2016 May 11. Nature. 2016. PMID: 27225113 No abstract available.
-
Nucleus to Mitochondria: Lost in Transcription, Found in Translation.Dev Cell. 2016 Jun 20;37(6):490-2. doi: 10.1016/j.devcel.2016.06.003. Dev Cell. 2016. PMID: 27326927
References
-
- Masters BS, Stohl LL, Clayton DA. Yeast mitochondrial RNA polymerase is homologous to those encoded by bacteriophages T3 and T7. Cell. 1987;51:89–99. - PubMed
-
- Faye G, Sor F. Analysis of mitochondrial ribosomal proteins of Saccharomyces cerevisiae by two dimensional polyacrylamide gel electrophoresis. Mol. Gen. Genet. 1977;155:27–34. - PubMed
-
- Kehrein K, Bonnefoy N, Ott M. Mitochondrial protein synthesis: efficiency and accuracy. Antioxidants & redox signaling. 2013;19:1928–1939. doi:10.1089/ars.2012.4896. - PubMed
-
- Costanzo MC, Fox TD. Control of mitochondrial gene expression in Saccharomyces cerevisiae. Annual review of genetics. 1990;24:91–113. doi:10.1146/annurev.ge.24.120190.000515. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

