Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer's disease
- PMID: 27225182
- PMCID: PMC5505565
- DOI: 10.1126/scitranslmed.aaf1059
Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer's disease
Abstract
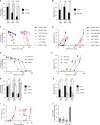
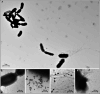
The amyloid-β peptide (Aβ) is a key protein in Alzheimer's disease (AD) pathology. We previously reported in vitro evidence suggesting that Aβ is an antimicrobial peptide. We present in vivo data showing that Aβ expression protects against fungal and bacterial infections in mouse, nematode, and cell culture models of AD. We show that Aβ oligomerization, a behavior traditionally viewed as intrinsically pathological, may be necessary for the antimicrobial activities of the peptide. Collectively, our data are consistent with a model in which soluble Aβ oligomers first bind to microbial cell wall carbohydrates via a heparin-binding domain. Developing protofibrils inhibited pathogen adhesion to host cells. Propagating β-amyloid fibrils mediate agglutination and eventual entrapment of unatttached microbes. Consistent with our model, Salmonella Typhimurium bacterial infection of the brains of transgenic 5XFAD mice resulted in rapid seeding and accelerated β-amyloid deposition, which closely colocalized with the invading bacteria. Our findings raise the intriguing possibility that β-amyloid may play a protective role in innate immunity and infectious or sterile inflammatory stimuli may drive amyloidosis. These data suggest a dual protective/damaging role for Aβ, as has been described for other antimicrobial peptides.
Copyright © 2016, American Association for the Advancement of Science.
Conflict of interest statement
Figures





Comment in
-
Host response: A trigger for neurodegeneration?Nat Microbiol. 2016 Jul 26;1(8):16129. doi: 10.1038/nmicrobiol.2016.129. Nat Microbiol. 2016. PMID: 27573123 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

