Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone
- PMID: 27225183
- PMCID: PMC8722465
- DOI: 10.1126/scitranslmed.aad4059
Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone
Abstract
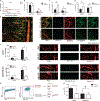
Breast cancer metastatic relapse can occur years after therapy, indicating that disseminated breast cancer cells (BCCs) have a prolonged dormant phase before becoming proliferative. A major site of disease dissemination and relapse is bone, although the critical signals that allow circulating BCCs to identify bone microvasculature, enter tissue, and tether to the microenvironment are poorly understood. Using real-time in vivo microscopy of bone marrow (BM) in a breast cancer xenograft model, we show that dormant and proliferating BCCs occupy distinct areas, with dormant BCCs predominantly found in E-selectin- and stromal cell-derived factor 1 (SDF-1)-rich perisinusoidal vascular regions. We use highly specific inhibitors of E-selectin and C-X-C chemokine receptor type 4 (CXCR4) (SDF-1 receptor) to demonstrate that E-selectin and SDF-1 orchestrate opposing roles in BCC trafficking. Whereas E-selectin interactions are critical for allowing BCC entry into the BM, the SDF-1/CXCR4 interaction anchors BCCs to the microenvironment, and its inhibition induces mobilization of dormant micrometastases into circulation. Homing studies with primary BCCs also demonstrate that E-selectin regulates their entry into bone through the sinusoidal niche, and immunohistochemical staining of patient BMs shows dormant micrometastatic disease adjacent to SDF-1(+) vasculature. These findings shed light on how BCCs traffic within the host, and suggest that simultaneous blockade of CXCR4 and E-selectin in patients could molecularly excise dormant micrometastases from the protective BM environment, preventing their emergence as relapsed disease.
Copyright © 2016, American Association for the Advancement of Science.
Figures



References
-
- DeVita VT Jr., Lawrence TS, Rosenberg SA, Eds., DeVita, Hellman, and Rosenberg’s Cancer: Principles & Practice of Oncology (Lippincott, Williams & Wilkins, Philadelphia, PA, 2014).
-
- Braun S, Vogl F, Naume B, Janni W, Osborne MP, Coombes RC, Schlimok G, Diel IJ, Gerber B, Gebauer G, Pierga J-Y, Marth C, Oruzio D, Wiedswang G, Solomayer E-F, Kundt G, Strobl B, Fehm T, Wong GYC, Bliss J, Vincent-Salomon A, Pantel K, A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med 353, 793–802 (2005). - PubMed
-
- Weigelt B, Peterse JL, van’t Veer LJ, Breast cancer metastasis: Markers and models. Nat. Rev. Cancer 5, 591–602 (2005). - PubMed
-
- Rack B, Schindlbeck C, Jückstock J, Andergassen U, Hepp P, Zwingers T, Friedl TWP, Lorenz R, Tesch H, Fasching PA, Fehm T, Schneeweiss A, Lichtenegger W, Beckmann MW, Friese K, Pantel K, Janni W; SUCCESS Study Group, Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J. Natl. Cancer Inst 106, dju066 (2013). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

